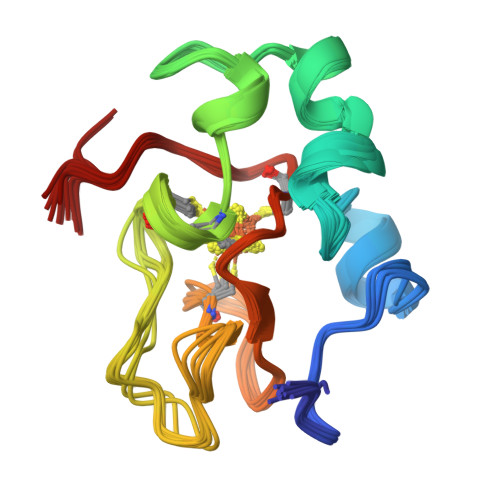
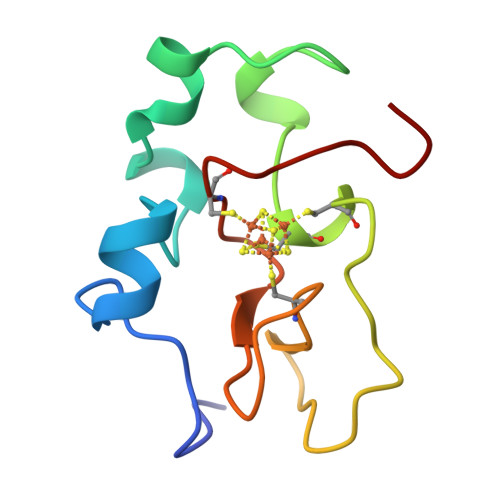
The three-dimensional solution structure of the reduced high-potential iron-sulfur protein from Chromatium vinosum through NMR.
Banci, L., Bertini, I., Dikiy, A., Kastrau, D.H., Luchinat, C., Sompornpisut, P.(1995) Biochemistry 34: 206-219
- PubMed: 7819198
- DOI: https://doi.org/10.1021/bi00001a025
- Primary Citation of Related Structures:
1HRQ, 1HRR - PubMed Abstract:
The 1H NMR assignment of the reduced HiPIP from Chromatium vinosum available in the literature [Gaillard, J., Albrand, J.-P., Moulis, J.-M., & Wemmer, D. E. (1992) Biochemistry 31, 5632-5639] has been extended up to 85% of the total protein protons. Ninety percent of the nitrogens have been assigned. Then the solution structure has been obtained using as many as 1147 meaningful NOE connectivities. The protein is sizably paramagnetic even though the ground state is a singlet. Nevertheless, the final RMSD values are 0.62 and 1.19 A for the backbone and the heavy atoms, respectively. These values compare well with those for diamagnetic proteins of the same size. The solution structure is discussed in the light of the available structural information from X-ray data.
Organizational Affiliation:
Department of Chemistry, University of Florence, Italy.