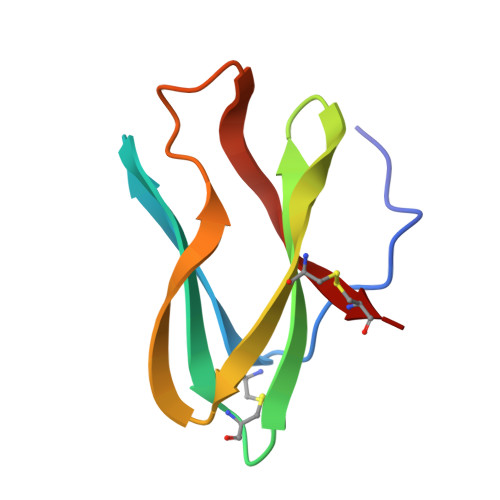
Crystal structure determination, refinement and the molecular model of the alpha-amylase inhibitor Hoe-467A.
Pflugrath, J.W., Wiegand, G., Huber, R., Vertesy, L.(1986) J Mol Biol 189: 383-386
- PubMed: 3489104
- DOI: https://doi.org/10.1016/0022-2836(86)90520-6
- Primary Citation of Related Structures:
1HOE - PubMed Abstract:
The crystal and molecular structure of the alpha-amylase inhibitor Hoe-467A has been determined and refined at high resolution. The polypeptide chain is folded in two triple-stranded sheets, which form a barrel. The topology of folding is as found in the immunoglobulin domains. The amino acid triplet Trp18-Arg19-Tyr20 has an exceptional conformation and position in the molecule and is possibly involved in inhibitory activity.