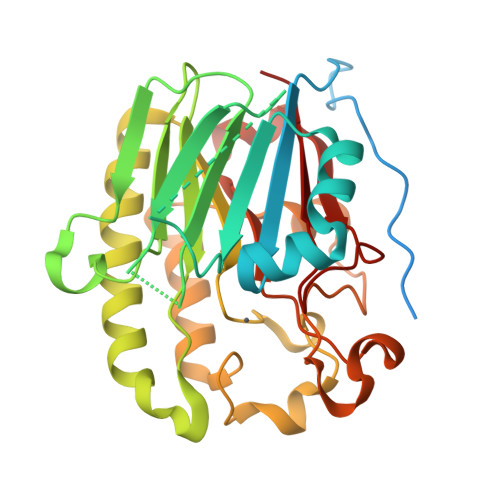
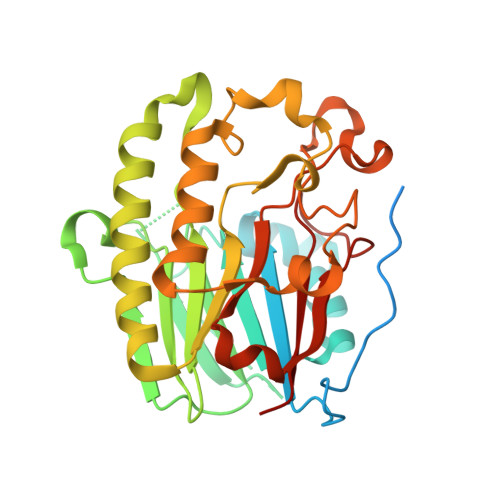
Two Divalent Metal Ions in the Active Site of a New Crystal Form of Human Apurinic/Apyrimidinic Endonuclease, Ape1: Implications for the Catalytic Mechanism
Beernink, P.T., Segelke, B.W., Hadi, M.Z., Erzberger, J.P., Wilson III, D.M., Rupp, B.(2001) J Mol Biology 307: 1023
- PubMed: 11286553
- DOI: https://doi.org/10.1006/jmbi.2001.4529
- Primary Citation of Related Structures:
1E9N, 1HD7 - PubMed Abstract:
The major human abasic endonuclease, Ape1, is an essential DNA repair enzyme that initiates the removal of apurinic/apyrimidinic sites from DNA, excises 3' replication-blocking moieties, and modulates the DNA binding activity of several transcriptional regulators. We have determined the X-ray structure of the full-length human Ape1 enzyme in two new crystal forms, one at neutral and one at acidic pH. The new structures are generally similar to the previously determined structure of a truncated Ape1 protein, but differ in the conformation of several loop regions and in spans of residues with weak electron density. While only one active-site metal ion is present in the structure determined at low pH, the structure determined from a crystal grown at the pH optimum of Ape1 nuclease activity, pH 7.5, has two metal ions bound 5 A apart in the active site. Enzyme kinetic data indicate that at least two metal-binding sites are functionally important, since Ca(2+) exhibits complex stimulatory and inhibitory effects on the Mg(2+)-dependent catalysis of Ape1, even though Ca(2+) itself does not serve as a cofactor. In conjunction, the structural and kinetic data suggest that Ape1 catalyzes hydrolysis of the DNA backbone through a two metal ion-mediated mechanism.
Organizational Affiliation:
Molecular and Structural Biology Division, Biology and Biotechnology Research Program, Lawrence Livermore National Laboratory, Livermore, CA, 94550, USA.