Asymmetric Conductivity of Engineered Porins
Bannwarth, M., Schulz, G.E.(2002) Protein Eng 15: 799
- PubMed: 12468713
- DOI: https://doi.org/10.1093/protein/15.10.799
- Primary Citation of Related Structures:
1H6S - PubMed Abstract:
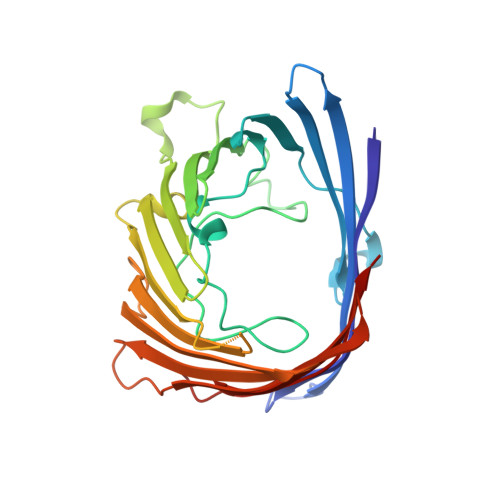
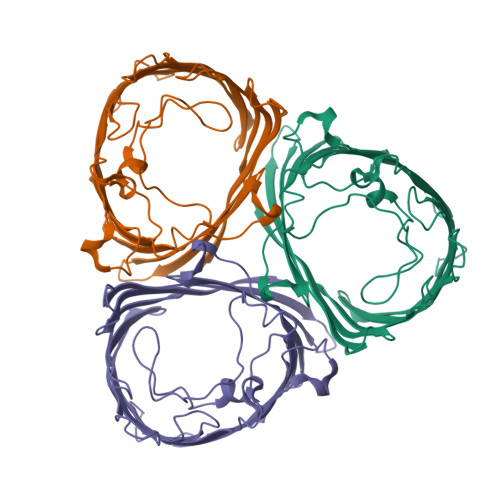
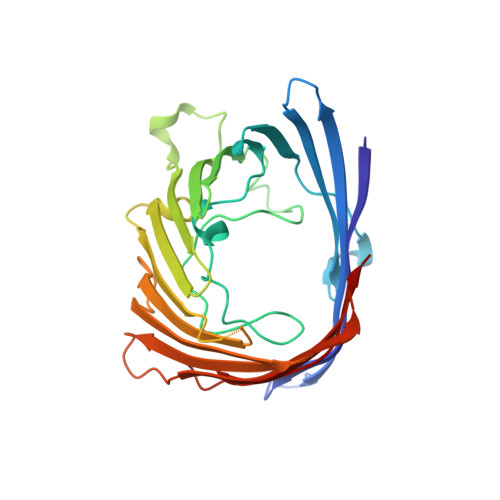
Positively charged peptide segments of 16 and 18 residues were inserted at a periplasmic turn of the porin from Rhodobacter blasticus in order to form an electric field-dependent plug. The X-ray diffraction analysis of a mutant confirmed that the structure of the porin had remained intact and that the insert was mobile. Incorporation experiments of single molecules into lipid bilayers showed that the distribution of electric conduction increments depended on the field polarity. The observed distributions are explained if the porin molecules enter the bilayer preferentially with their periplasmic surface first. Furthermore, the conduction of membrane-incorporated porin mutants changed reproducibly on field reversal showing asymmetries of reverse similar 15%, while the wild-type remained constant. This asymmetry is most likely caused by the electric field pressing the charged insert onto the pore eyelet in one field direction and removing it from the eyelet in the other. The results encourage attempts to improve the inserts in order to eventually reach diode characteristics.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie, Albert-Ludwigs-Universität, Albertstrasse 21, D-79104 Freiburg im Breisgau, Germany.