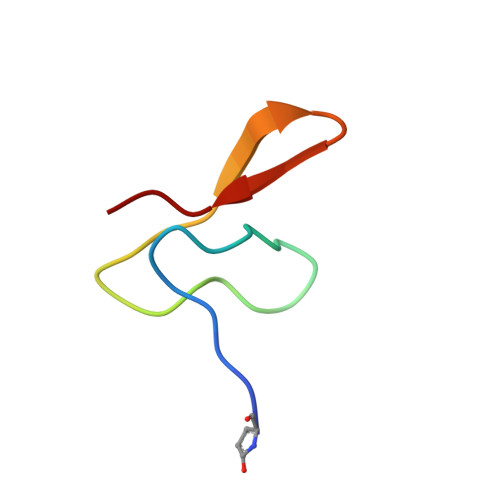
Three-dimensional structure of gurmarin, a sweet taste-suppressing polypeptide.
Arai, K., Ishima, R., Morikawa, S., Miyasaka, A., Imoto, T., Yoshimura, S., Aimoto, S., Akasaka, K.(1995) J Biomol NMR 5: 297-305
- PubMed: 7787425
- DOI: https://doi.org/10.1007/BF00211756
- Primary Citation of Related Structures:
1GUR - PubMed Abstract:
The solution structure of gurmarin was studied by two-dimensional proton NMR spectroscopy at 600 MHz. Gurmarin, a 35-amino acid residue polypeptide recently discovered in an Indian-originated tree Gymnema sylvestre, selectively suppresses the neural responses of rat to sweet taste stimuli. Sequence-specific resonance assignments were obtained for all backbone protons and for most of the side-chain protons. The three-dimensional solution structure was determined by simulated-annealing calculations on the basis of 135 interproton distance constraints derived from NOEs, six distance constraints for three hydrogen bonds and 16 dihedral angle constraints derived from coupling constants. A total of 10 structures folded into a well-defined structure with a triple-stranded antiparallel beta-sheet. The average rmsd values between any two structures were 1.65 +/- 0.39 A for the backbone atoms (N, C alpha, C) and 2.95 +/- 0.27 A for all heavy atoms. The positions of the three disulfide bridges, which could not be determined chemically, were estimated to be Cys3-Cys18, Cys10-Cys23 and Cys17-Cys33 on the basis of the NMR distance constraints. This disulfide bridge pattern in gurmarin turned out to be analogous to that in omega-conotoxin and Momordica charantia trypsin inhibitor-II, and the topology of folding was the same as that in omega-conotoxin.
Organizational Affiliation:
Department of Chemistry, Faculty of Science, Kyoto University, Japan.