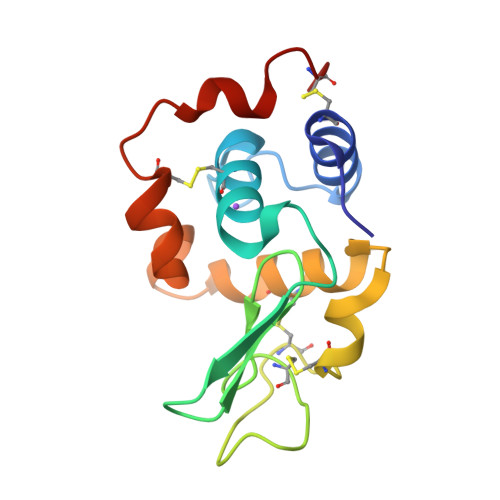
Role of non-glycine residues in left-handed helical conformation for the conformational stability of human lysozyme
Takano, K., Yamagata, Y., Yutani, K.(2001) Proteins 44: 233-243
- PubMed: 11455596
- DOI: https://doi.org/10.1002/prot.1088
- Primary Citation of Related Structures:
1GDW, 1GDX, 1GE0, 1GE1, 1GE2, 1GE3, 1GE4 - PubMed Abstract:
To understand the role of non-Gly residues in the left-handed helical conformation for the conformational stability of a protein, the non-Gly to Gly and Ala mutations at six left-handed residues (R21, Y38, R50, Q58, H78, and N118) of the human lysozyme were examined. The thermodynamic parameters for denaturation were determined using a differential scanning calorimeter, and the crystal structures were analyzed by X-ray crystallography. If a left-handed non-Gly had an unfavorable steric interaction between the side-chain Cbeta and backbone, the Gly mutation would be expected to stabilize more than the Ala mutation at the same position. For the mutant human lysozymes, however, there were few differences in the denaturation Gibbs energy (DeltaG) between the Gly and Ala mutants, except for the substitution at position 58. Analysis of the changes in stability (DeltaDeltaG) based on the structures of the wild-type and mutant proteins showed that the experimental DeltaDeltaG value of Q58G was approximately 7 kJ/mol higher than the estimated value without consideration of any local steric interaction. These results indicate that only Q58G increased the stability by elimination of local constraints. The residue 58 is located at the most rigid position in the left-handed non-Gly residues and is involved in its enzymatic function. It can be concluded that the left-handed non-Gly residues do not always have unfavorable strain energies as compared with Gly at the same position.
Organizational Affiliation:
Institute for Protein Research, Osaka University, Yamadaoka, Suita, Osaka, Japan.