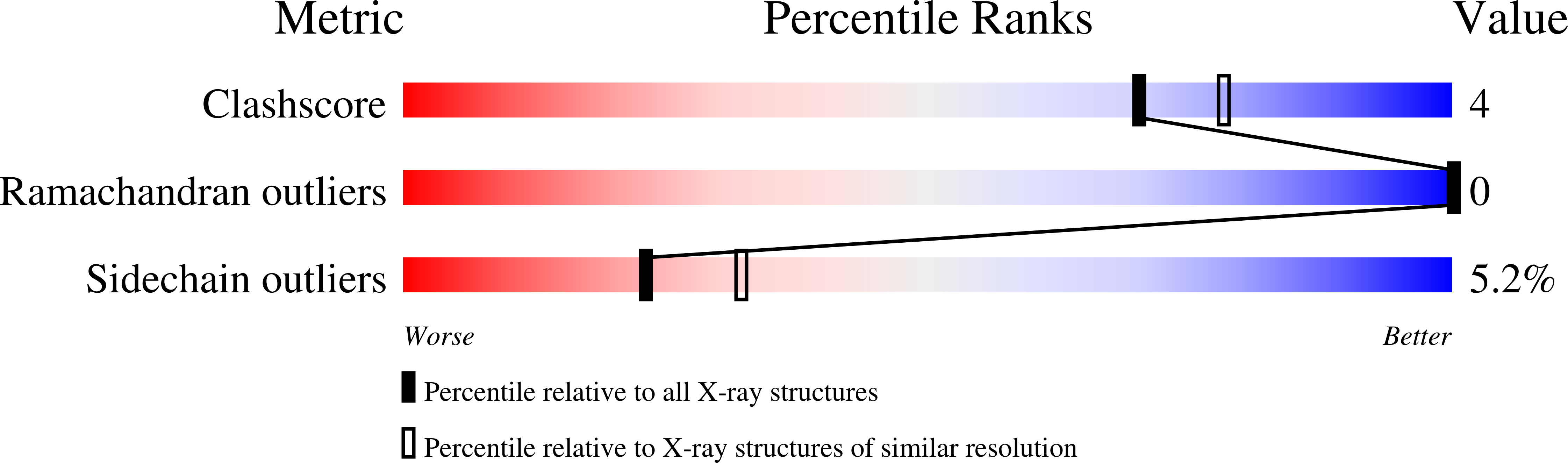Structure at pH 6.5 of ferredoxin I from Azotobacter vinelandii at 2.3 A resolution.
Merritt, E.A., Stout, G.H., Turley, S., Sieker, L.C., Jehsen, L.H., Orme-Johnson, W.H.(1993) Acta Crystallogr D Biol Crystallogr 49: 272-281
- PubMed: 15299532
- DOI: https://doi.org/10.1107/S0907444992007248
- Primary Citation of Related Structures:
1FER - PubMed Abstract:
Ferredoxin I from Azotobacter vinelandii (AvFdI) is an iron-sulfur protein composed of 106 amino acids, seven Fe atoms and eight inorganic S* atoms. A crystallographic redetermination of its structure showed the originally reported structure to be incorrect. We report here the crystal structure of AvFdI at pH 6.5. Extensive refinement has led to a final R value of 0.170 for all 6986 non-extinct reflections in the range 10-2.3 A using a solvent model which includes 98 discrete solvent atoms with occupancies between 0.3 and 1.0 and an average B value of 22.5 A(2). The first half of the peptide chain closely resembles that of the 55-residue ferredoxin from Peptococcus aerogenes (PaFd), while the remainder consists of three turns of helix and a series of loops which form a cap over part of the molecular core. Despite the similarities in structure and surroundings, the corresponding 4Fe4S* clusters in PaFd and AvFdI have strikingly different redox potentials; a possible explanation has been sought in the differing hydration models for the two molecules.
Organizational Affiliation:
Department of Biological Structure, University of Washington, Seattle 98195, USA.