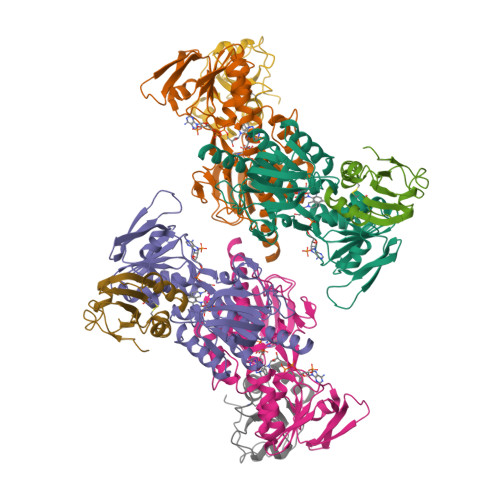
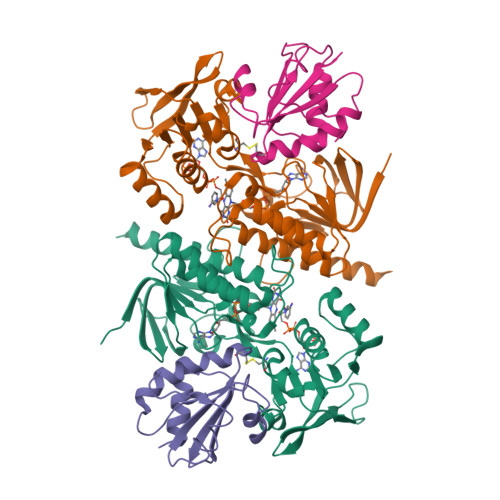
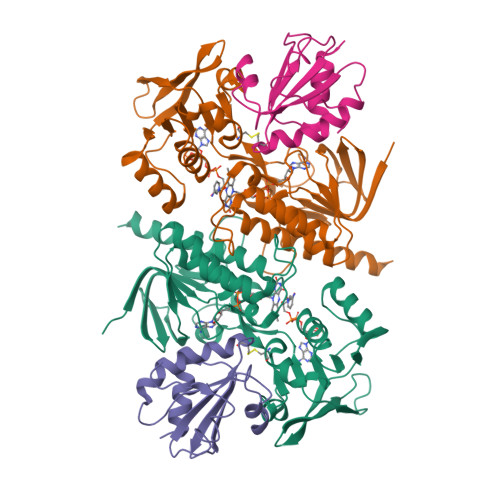
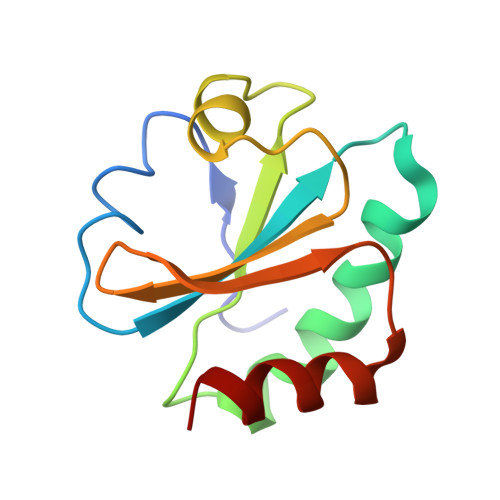
Twists in catalysis: alternating conformations of Escherichia coli thioredoxin reductase.
Lennon, B.W., Williams Jr., C.H., Ludwig, M.L.(2000) Science 289: 1190-1194
- PubMed: 10947986
- DOI: https://doi.org/10.1126/science.289.5482.1190
- Primary Citation of Related Structures:
1F6M - PubMed Abstract:
In thioredoxin reductase (TrxR) from Escherichia coli, cycles of reduction and reoxidation of the flavin adenine dinucleotide (FAD) cofactor depend on rate-limiting rearrangements of the FAD and NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) domains. We describe the structure of the flavin-reducing conformation of E. coli TrxR at a resolution of 3.0 angstroms. The orientation of the two domains permits reduction of FAD by NADPH and oxidation of the enzyme dithiol by the protein substrate, thioredoxin. The alternate conformation, described by Kuriyan and co-workers, permits internal transfer of reducing equivalents from reduced FAD to the active-site disulfide. Comparison of these structures demonstrates that switching between the two conformations involves a "ball-and-socket" motion in which the pyridine nucleotide-binding domain rotates by 67 degrees.
Organizational Affiliation:
Biophysics Research Division, Department of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109, USA.