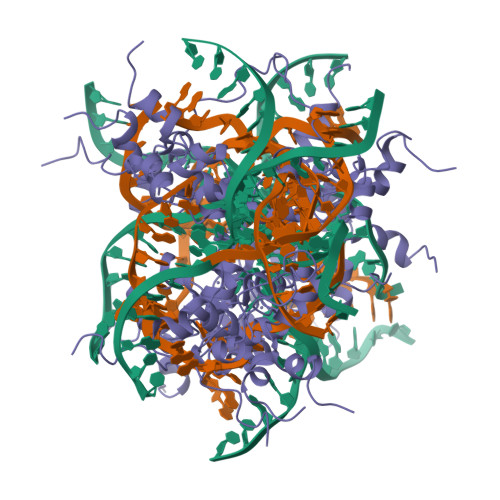
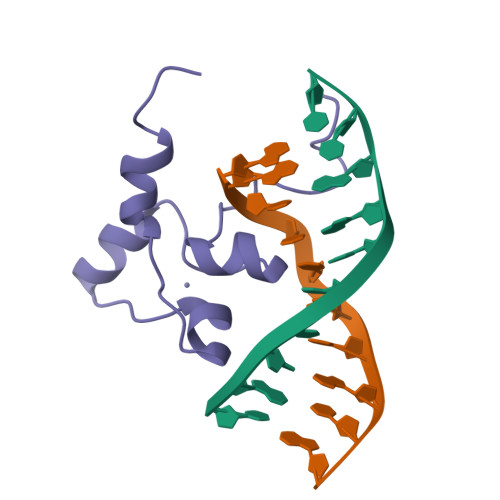
The solution structure of an AlcR-DNA complex sheds light onto the unique tight and monomeric DNA binding of a Zn(2)Cys(6) protein.
Cahuzac, B., Cerdan, R., Felenbok, B., Guittet, E.(2001) Structure 9: 827-836
- PubMed: 11566132
- DOI: https://doi.org/10.1016/s0969-2126(01)00640-2
- Primary Citation of Related Structures:
1F4S, 1F5E - PubMed Abstract:
In Aspergillus nidulans, the transcription activator AlcR mediates specific induction of a number of the genes of the alc cluster. This cluster includes genes involved in the oxidation of ethanol and other alcohols to acetate. The pattern of binding and of transactivation of AlcR is unique within the Zn(2)Cys(6) family. The structural bases for these specificities have not been analyzed at the atomic level until now. We have used NMR spectroscopy and restrained molecular dynamics to determine a set of structures of the AlcR DNA binding domain [AlcR(1-60)] in complex with a 10-mer DNA duplex. Analysis of the structures reveals specific interactions between AlcR and DNA common to the other known zinc clusters. In addition, the involvement of the N-terminal residues upstream of the AlcR zinc cluster in DNA binding is clearly highlighted, and the pivotal role of R6 is confirmed. Totally unprecedented specific and nonspecific contacts of two additional regions of the protein with the DNA are demonstrated. The differences with the available crystallographic structures of other zinc binuclear cluster proteins-DNA complexes are analyzed. The structures of the AlcR(1-60)-DNA complex provide the basis for a better understanding of some of the specificities of the AlcR system: the DNA consensus recognition sequence--usually the triplet CGG--is extended to five base pairs, AlcR acts as a monomer, and additional contacts inside and outside the DNA binding domain in the major and minor groove are observed. These extensive interactions stabilize the AlcR monomer to its cognate DNA site.
Organizational Affiliation:
Laboratoire de Résonance Magnétique Nucléaire, ICSN-CNRS, 1 Avenue de la Terrasse, Gif-sur-Yvette F-91190, France.