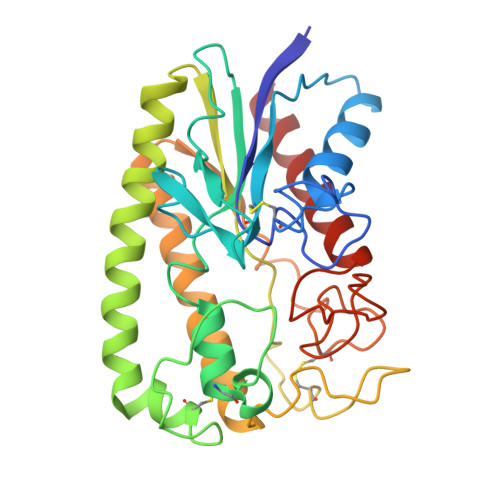
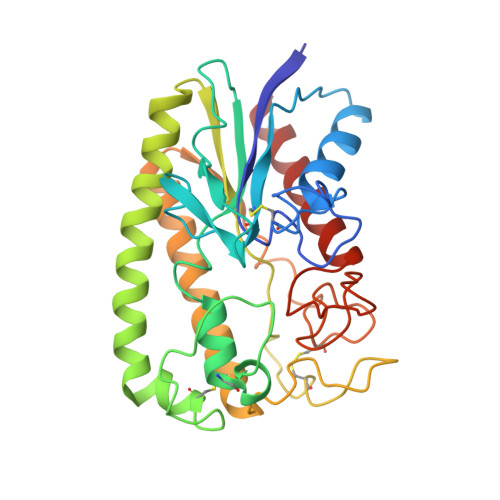
A novel variant of the catalytic triad in the Streptomyces scabies esterase.
Wei, Y., Schottel, J.L., Derewenda, U., Swenson, L., Patkar, S., Derewenda, Z.S.(1995) Nat Struct Biol 2: 218-223
- PubMed: 7773790
- DOI: https://doi.org/10.1038/nsb0395-218
- Primary Citation of Related Structures:
1ESC, 1ESD, 1ESE - PubMed Abstract:
The crystal structure of a novel esterase from Streptomyces scabies, a causal agent of the potato scab disease, was solved at 2.1 A resolution. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases. The active site contains a dyad of Ser 14 and His 283, closely resembling two of the three components of typical Ser-His-Asp(Glu) triads from other serine hydrolases. Proper orientation of the active site imidazol is maintained by a hydrogen bond between the N delta-H group and a main chain oxygen. Thus, the enzyme constitutes the first known natural variation of the chymotrypsin-like triad in which a carboxylic acid is replaced by a neutral hydrogen-bond acceptor.
Organizational Affiliation:
Medical Research Council of Canada Group in Protein Structure and Function, Department of Biochemistry, University of Alberta, Edmonton.