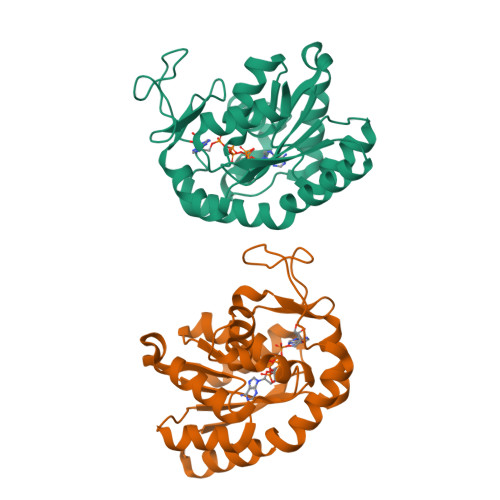
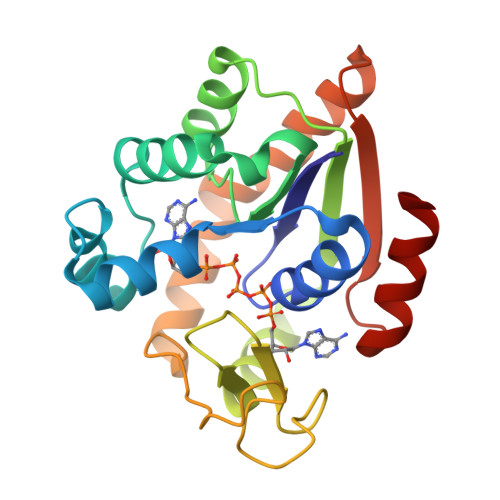
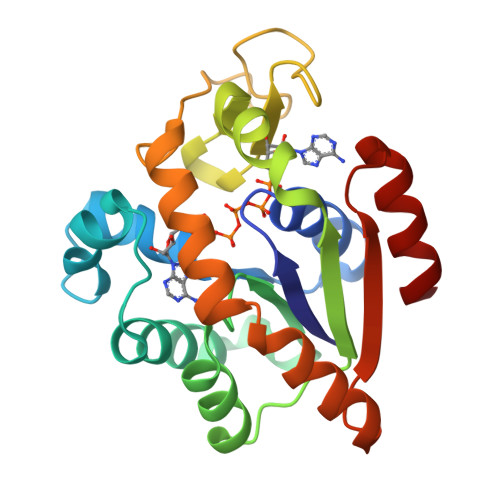
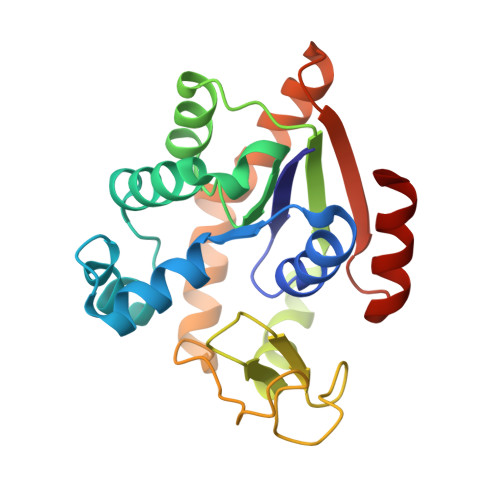
Crystal structures of two mutants of adenylate kinase from Escherichia coli that modify the Gly-loop.
Muller, C.W., Schulz, G.E.(1993) Proteins 15: 42-49
- PubMed: 8451239
- DOI: https://doi.org/10.1002/prot.340150106
- Primary Citation of Related Structures:
1E4V, 1E4Y - PubMed Abstract:
Two mutants of adenylate kinase from Escherichia coli have been crystallized and analyzed by X-ray diffraction at resolutions of 3.4 and 2.4 A, respectively. These mutants are Pro-9-->Leu and Gly-10-->Val. They were selected for their positions in the highly conserved Gly-loop forming a giant anion hole for the beta-phosphate of ATP (GTP) in adenylate kinases, H-ras-p21, and other nucleotide-binding proteins. Mutants at these positions of H-ras-p21 cause cancer. In adenylate kinase these mutations cause smallish changes at the active site. Relating the structural changes to the known changes in catalysis indicates that these mutants hinder the induced-fit movements. As a side result we find that mutant Pro-9-->Leu and wild-type form one very similar crystal packing contact that is crystallographic in one case and noncrystallographic in the other, while all other packing contacts and the space groups are quite at variance.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie der Universität, Freiburg im Breisgau, Federal Republic of Germany.