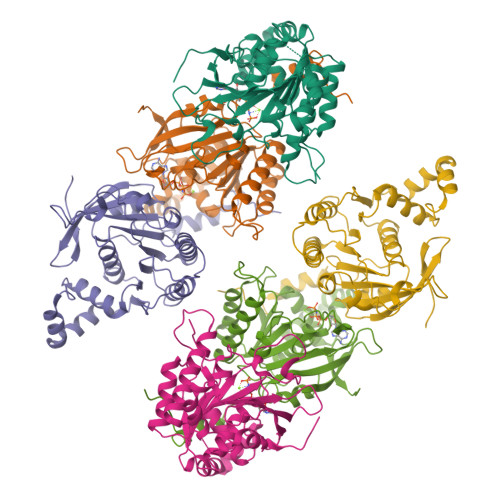
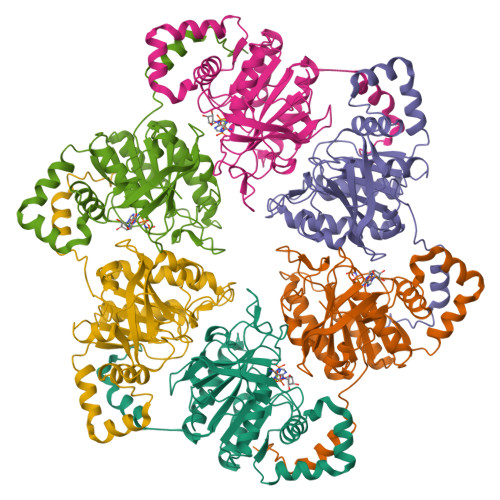
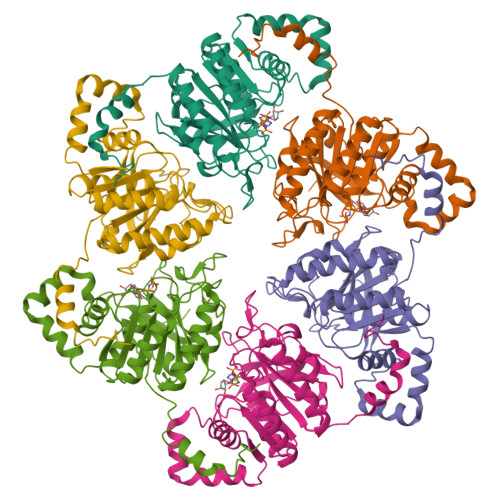
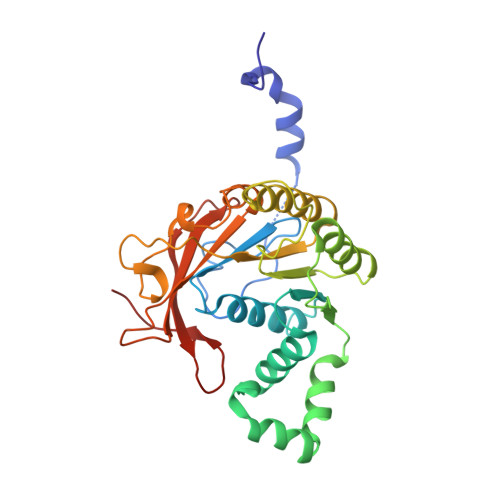
Crystal Structure of T7 Gene 4 Ring Helicase Indicates a Mechanism for Sequential Hydrolysis of Nucleotides
Singleton, M.R., Sawaya, M.R., Ellenberger, T., Wigley, D.B.(2000) Cell 101: 589
- PubMed: 10892646
- DOI: https://doi.org/10.1016/s0092-8674(00)80871-5
- Primary Citation of Related Structures:
1E0J, 1E0K - PubMed Abstract:
We have determined the crystal structure of an active, hexameric fragment of the gene 4 helicase from bacteriophage T7. The structure reveals how subunit contacts stabilize the hexamer. Deviation from expected six-fold symmetry of the hexamer indicates that the structure is of an intermediate on the catalytic pathway. The structural consequences of the asymmetry suggest a "binding change" mechanism to explain how cooperative binding and hydrolysis of nucleotides are coupled to conformational changes in the ring that most likely accompany duplex unwinding. The structure of a complex with a nonhydrolyzable ATP analog provides additional evidence for this hypothesis, with only four of the six possible nucleotide binding sites being occupied in this conformation of the hexamer. This model suggests a mechanism for DNA translocation.
Organizational Affiliation:
Sir William Dunn School of Pathology, University of Oxford, United Kingdom.