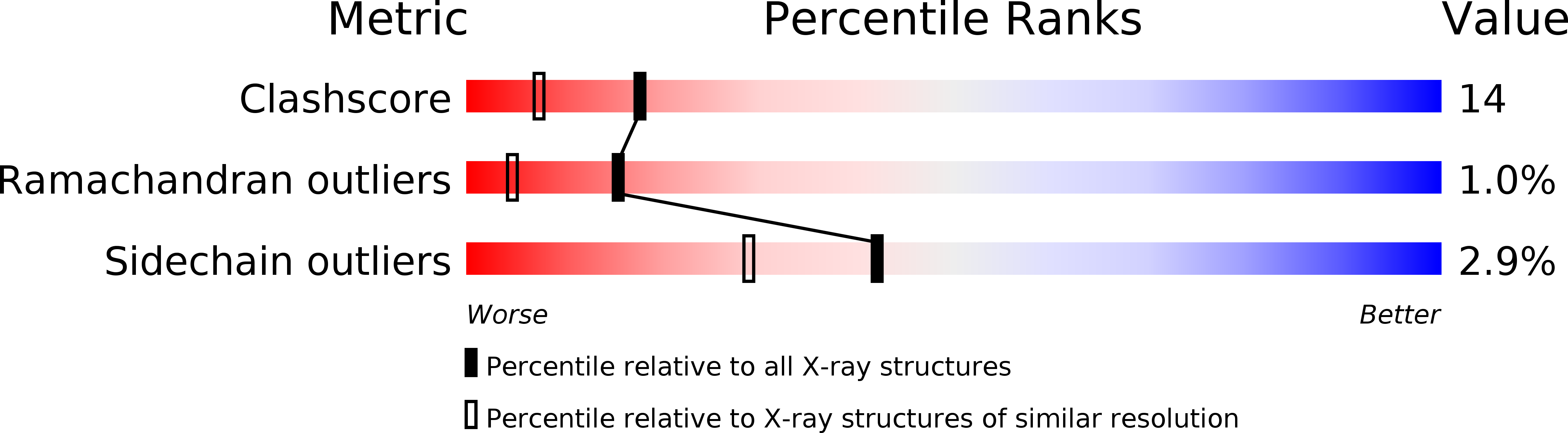Structure-based experimental confirmation of biochemical function to a methyltransferase, MJ0882, from hyperthermophile Methanococcus jannaschii
Huang, L., Hung, L., Odell, M., Yokota, H., Kim, R., Kim, S.H.(2002) J Struct Funct Genomics 2: 121-127
- PubMed: 12836702
- DOI: https://doi.org/10.1023/a:1021279113558
- Primary Citation of Related Structures:
1DUS - PubMed Abstract:
We have determined the three-dimensional (3-D) structure of protein MJ0882, which derives from a hypothetical open reading frame in the genome of the hyperthermophile Methanococcus jannaschii. The 3-D fold of MJ0882 at 1.8 A highly resembles that of a methyltransferase, despite limited sequence similarity to any confirmed methyltransferase. The structure has an S-adenosylmethionine (AdoMet) binding pocket surrounded by motifs with similarities to those commonly found among AdoMet binding proteins. Preliminary biochemical experiments show that MJ0882 specifically binds to AdoMet, which is the essential co-factor for methyltransferases.
Organizational Affiliation:
Dept. of Cellular Biochemistry and Biophysics, Sloan Kettering Institute, 1275 York Ave., New York, NY 10021, USA.