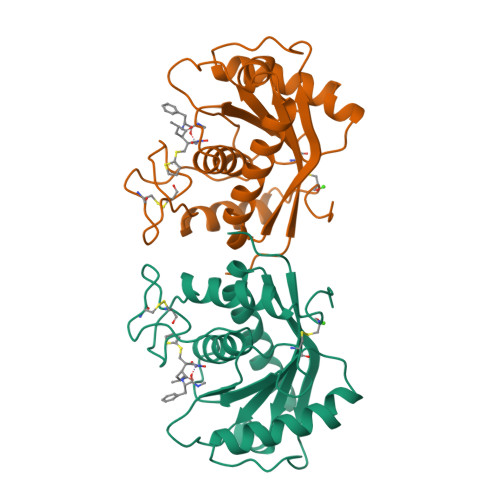
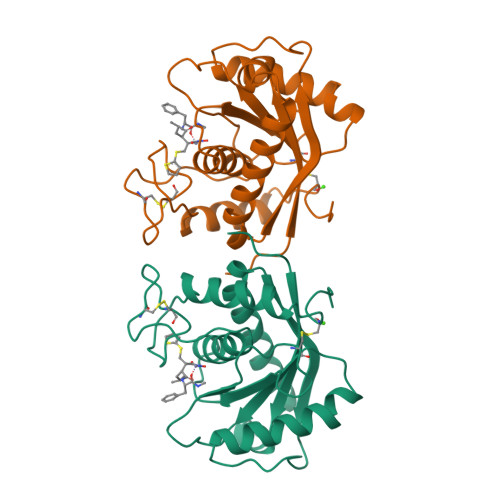
Batimastat, a potent matrix mealloproteinase inhibitor, exhibits an unexpected mode of binding.
Botos, I., Scapozza, L., Zhang, D., Liotta, L.A., Meyer, E.F.(1996) Proc Natl Acad Sci U S A 93: 2749-2754
- PubMed: 8610113
- DOI: https://doi.org/10.1073/pnas.93.7.2749
- Primary Citation of Related Structures:
1DTH - PubMed Abstract:
Matrix metalloproteinase enzymes have been implicated in degenerative processes like tumor cell invasion, metastasis, and arthritis. Specific metalloproteinase inhibitors have been used to block tumor cell proliferation. We have examined the interaction of batimastat (BB-94) with a metalloproteinase [atrolysin C (Ht-d), EC 3.4.24.42] active site at 2.0-angstroms resolution (R = 16.8%). The title structure exhibits an unexpected binding geometry, with the thiophene ring deeply inserted into the primary specificity site. This unprecedented binding geometry dramatizes the significance of the cavernous primary specificity site, pointing the way for the design of a new generation of potential antitumor drugs.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station, 77843, USA.