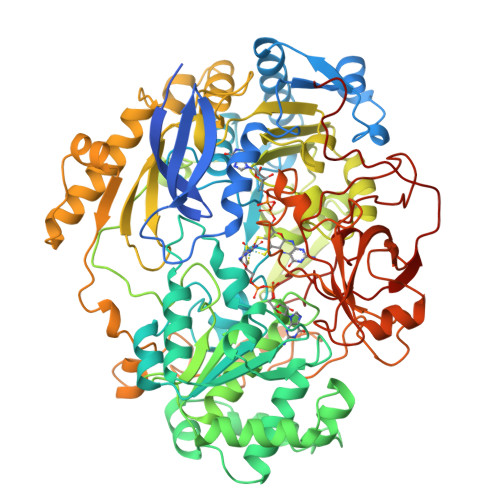
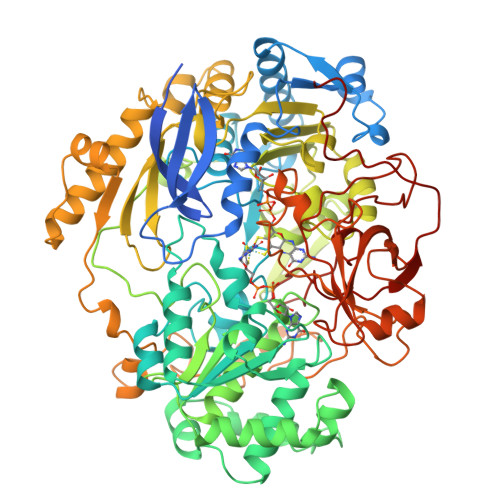
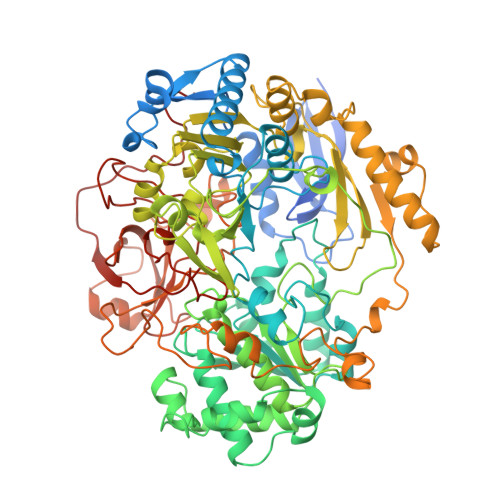
Molybdenum Active Centre of Dmso Reductase from Rhodobacter Capsulatus: Crystal Structure of the Oxidised Enzyme at 1.82-A Resolution and the Dithionite-Reduced Enzyme at 2.8-A Resolution
Mcalpine, A.S., Mcewan, A.G., Shaw, A., Bailey, S.(1997) J Biol Inorg Chem 2: 690-701