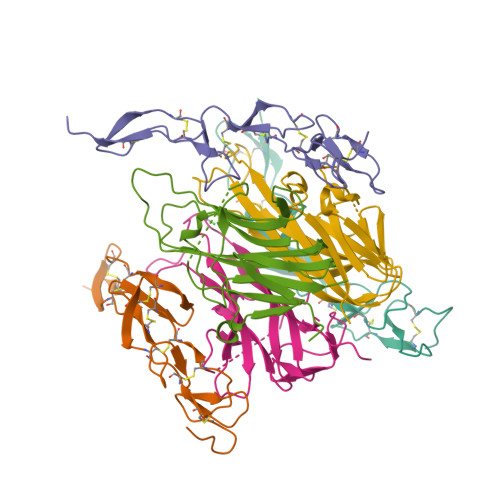
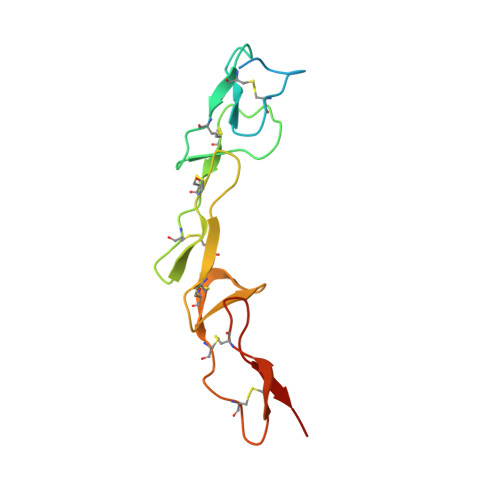
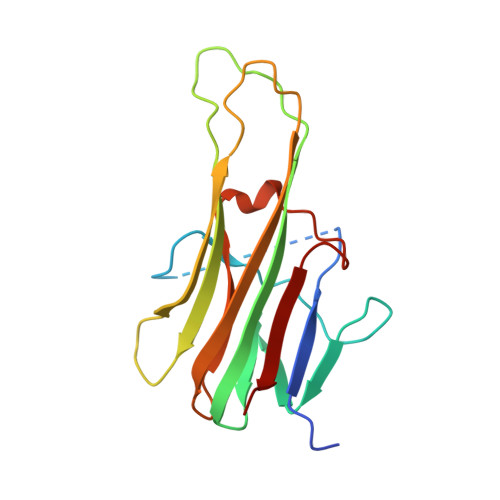
Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5.
Hymowitz, S.G., Christinger, H.W., Fuh, G., Ultsch, M., O'Connell, M., Kelley, R.F., Ashkenazi, A., de Vos, A.M.(1999) Mol Cell 4: 563-571
- PubMed: 10549288
- DOI: https://doi.org/10.1016/s1097-2765(00)80207-5
- Primary Citation of Related Structures:
1D0G - PubMed Abstract:
Formation of a complex between Apo2L (also called TRAIL) and its signaling receptors, DR4 and DR5, triggers apoptosis by inducing the oligomerization of intracellular death domains. We report the crystal structure of the complex between Apo2L and the ectodomain of DR5. The structure shows three elongated receptors snuggled into long crevices between pairs of monomers of the homotrimeric ligand. The interface is divided into two distinct patches, one near the bottom of the complex close to the receptor cell surface and one near the top. Both patches contain residues that are critical for high-affinity binding. A comparison to the structure of the lymphotoxin-receptor complex suggests general principles of binding and specificity for ligand recognition in the TNF receptor superfamily.
Organizational Affiliation:
Department of Protein Engineering, Genentech, Inc., South San Francisco, California 94080, USA.