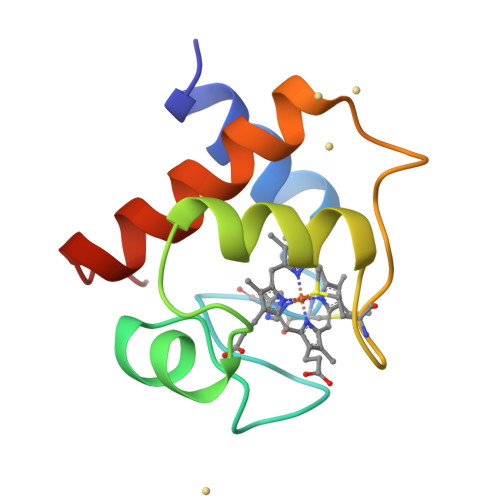
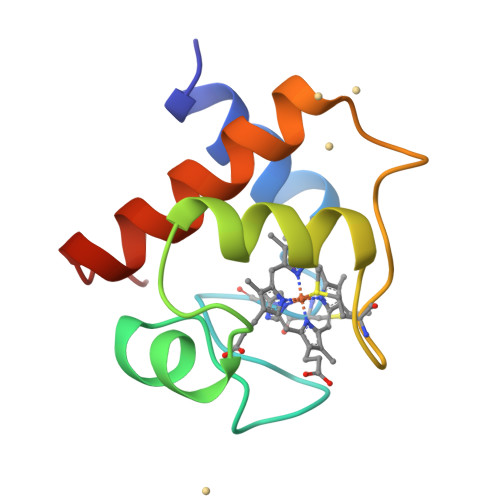
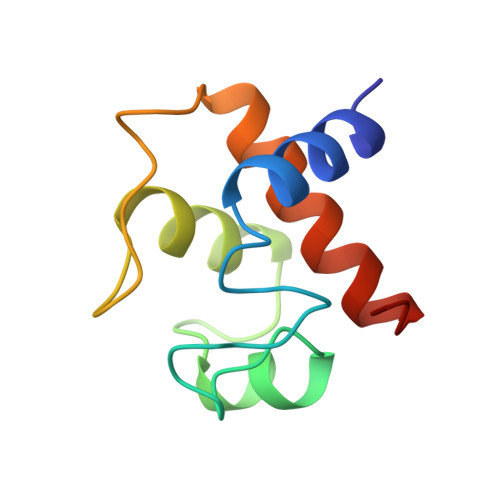
The structure of chloroplast cytochrome c6 at 1.9 A resolution: evidence for functional oligomerization.
Kerfeld, C.A., Anwar, H.P., Interrante, R., Merchant, S., Yeates, T.O.(1995) J Mol Biology 250: 627-647
- PubMed: 7623381
- DOI: https://doi.org/10.1006/jmbi.1995.0404
- Primary Citation of Related Structures:
1CYI, 1CYJ - PubMed Abstract:
The molecular structure of cytochrome c6 from the green alga Chlamydomonas reinhardtii has been determined from two crystal forms and refined to 1.9 A resolution. The two crystal forms are likely the result of different levels of post-translational modification of the protein. This is the first report of a high-resolution structure of a chloroplast-derived class I c-type cytochrome. The overall fold is similar to that of other class I c-type cytochromes, consisting of a series of alpha-helices and turns that envelop the heme prosthetic group. There is also a short two-stranded anti-parallel beta-sheet in the vicinity of the methionine axial ligand to the heme; this region of the molecule is formed by the most highly conserved residues in c6-type cytochromes. Although class I c-type cytochromes are assumed to function as monomers, both crystal forms of cytochrome c6 exhibit oligomerization about the heme crevice that is, in part, mediated by the short anti-parallel beta-sheet. The functional significance of this oligomerization is supported by the appearance of similar interfaces in other electron transfer couples, HPLC and light-scattering data, and is furthermore consistent with kinetic data on electron transfer reactions of c6-type cytochromes.
Organizational Affiliation:
Molecular Biology Institute, University of California, Los Angeles 90024-1570, USA.