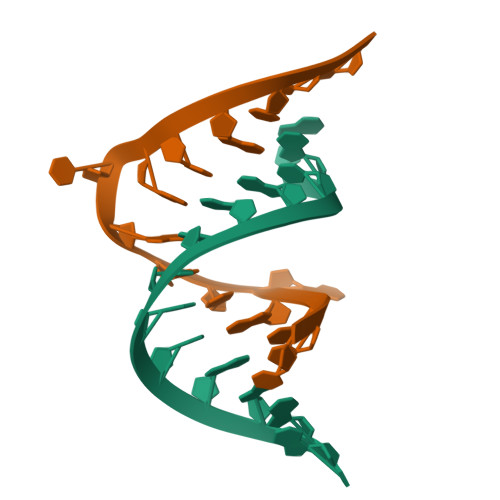
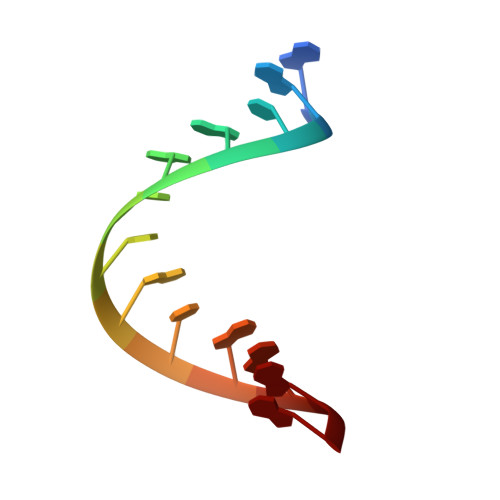
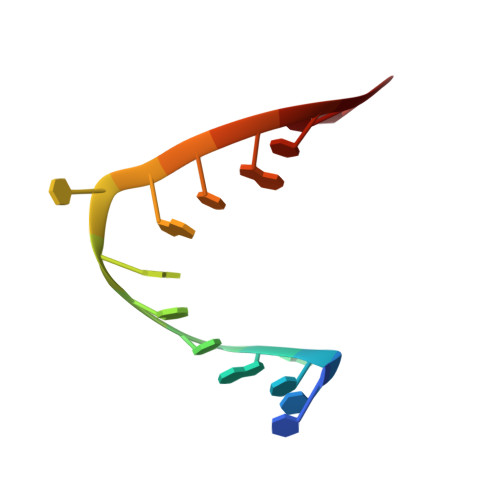
The structure of the HIV-1 RRE high affinity rev binding site at 1.6 A resolution.
Ippolito, J.A., Steitz, T.A.(2000) J Mol Biology 295: 711-717
- PubMed: 10656783
- DOI: https://doi.org/10.1006/jmbi.1999.3405
- Primary Citation of Related Structures:
1CSL - PubMed Abstract:
The crystal structure of a 28 nt RNA fragment containing the human immunodeficiency virus type 1 (HIV-1) Rev response element high affinity binding site for Rev protein has been solved at 1.6 A resolution. The overall structure of the RRE helix is greatly distorted from A-form geometry by the presence of two purine-purine base-pairs and two single nucleotide bulges. G48 and G71 form a Hoogsteen-type asymmetric base-pair with G71 adopting a syn conformation. The non-canonical regions in the unliganded Rev response element molecule narrow the major groove width with respect to standard A-RNA. The Rev response element structure observed here represents a closed form of the Rev binding site and differs from conformations of the RNA observed previously by solution NMR studies.
Organizational Affiliation:
Department of Molecular Biophysics, Howard Hughes Medical Institute, New Haven, CT 06520-8114, USA.