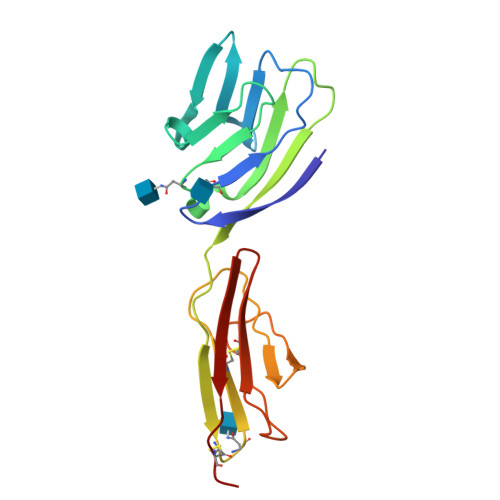
Crystal structure of the CD2-binding domain of CD58 (lymphocyte function-associated antigen 3) at 1.8-A resolution.
Ikemizu, S., Sparks, L.M., van der Merwe, P.A., Harlos, K., Stuart, D.I., Jones, E.Y., Davis, S.J.(1999) Proc Natl Acad Sci U S A 96: 4289-4294
- PubMed: 10200255
- DOI: https://doi.org/10.1073/pnas.96.8.4289
- Primary Citation of Related Structures:
1CCZ - PubMed Abstract:
The binding of the cell surface molecule CD58 (formerly lymphocyte function-associated antigen 3) to its ligand, CD2, significantly increases the sensitivity of antigen recognition by T cells. This was the first heterophilic cell adhesion interaction to be discovered and is now an important paradigm for analyzing the structural basis of cell-cell recognition. The crystal structure of a CD2-binding chimeric form of CD58, solved to 1.8-A resolution, reveals that the ligand binding domain of CD58 has the expected Ig superfamily V-set topology and shares several of the hitherto unique structural features of CD2, consistent with previous speculation that the genes encoding these molecules arose via duplication of a common precursor. Nevertheless, evidence for considerable divergence of CD2 and CD58 is also implicit in the structures. Mutations that disrupt CD2 binding map to the highly acidic surface of the AGFCC'C" beta-sheet of CD58, which, unexpectedly, lacks marked shape complementarity to the equivalent, rather more basic CD58-binding face of human CD2. The specificity of the very weak interactions of proteins mediating cell-cell recognition may often derive largely from electrostatic complementarity, with shape matching at the protein-protein interface being less exact than for interactions that combine specificity with high affinity, such as those involving antibodies.
Organizational Affiliation:
Laboratory of Molecular Biophysics, the Rex Richards Building, South Parks Road, Oxford, OX1 3QU, United Kingdom.