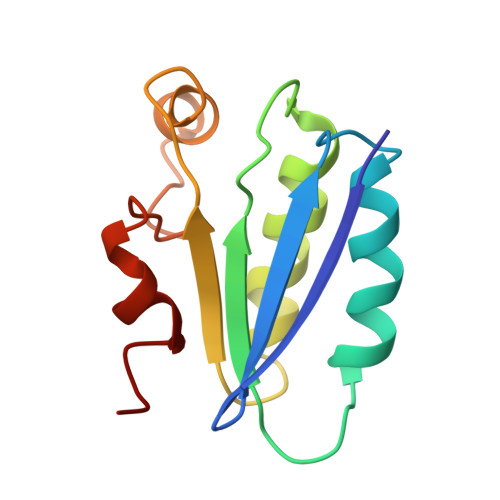
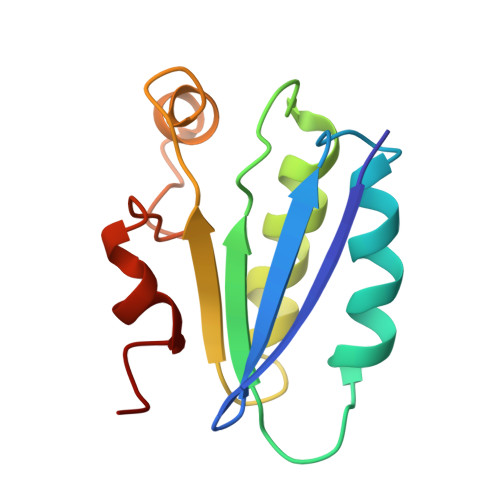
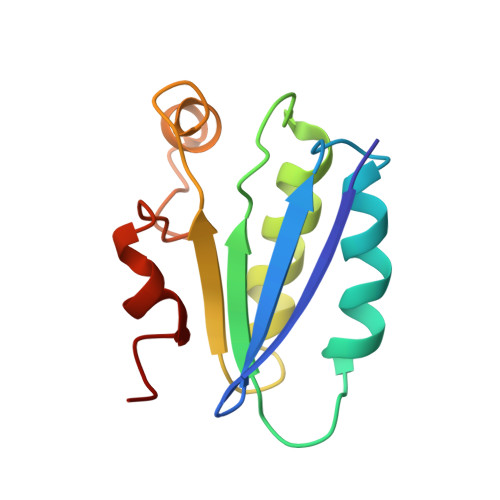
Solution structure of SpoIIAA, a phosphorylatable component of the system that regulates transcription factor sigmaF of Bacillus subtilis.
Kovacs, H., Comfort, D., Lord, M., Campbell, I.D., Yudkin, M.D.(1998) Proc Natl Acad Sci U S A 95: 5067-5071
- PubMed: 9560229
- DOI: https://doi.org/10.1073/pnas.95.9.5067
- Primary Citation of Related Structures:
1AUZ, 1BUZ - PubMed Abstract:
The establishment of differential gene expression in sporulating Bacillus subtilis involves four protein components, one of which, SpoIIAA, undergoes phosphorylation and dephosphorylation. We have used NMR spectroscopy to determine the solution structure of the nonphosphorylated form of SpoIIAA. The structure shows a fold consisting of a four-stranded beta-sheet and four alpha-helices. Knowledge of the structure helps to account for the phenotype of several strains of B. subtilis that carry known spoIIAA mutations and should facilitate investigations of the conformational consequences of phosphorylation.
Organizational Affiliation:
Department of Biochemistry, Department of Biochemistry, South Parks Road, Oxford University, Oxford OX1 3QU, United Kingdom.