Peptide based interleukin-1 beta converting enzyme (ICE) inhibitors: synthesis, structure activity relationships and crystallographic study of the ICE-inhibitor complex.
Okamoto, Y., Anan, H., Nakai, E., Morihira, K., Yonetoku, Y., Kurihara, H., Sakashita, H., Terai, Y., Takeuchi, M., Shibanuma, T., Isomura, Y.(1999) Chem Pharm Bull (Tokyo) 47: 11-21
- PubMed: 9987822
- DOI: https://doi.org/10.1248/cpb.47.11
- Primary Citation of Related Structures:
1BMQ - PubMed Abstract:
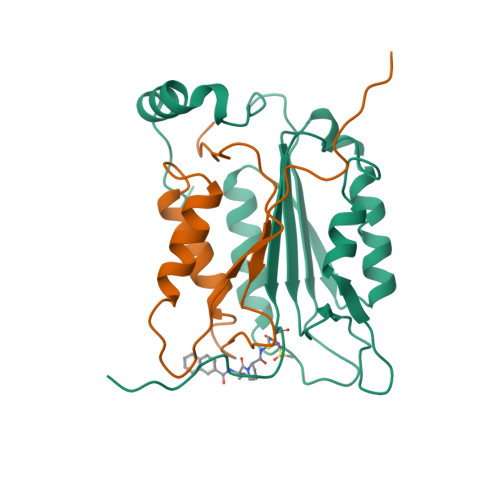
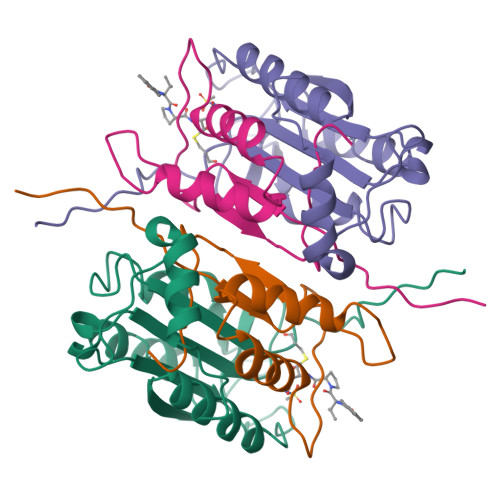
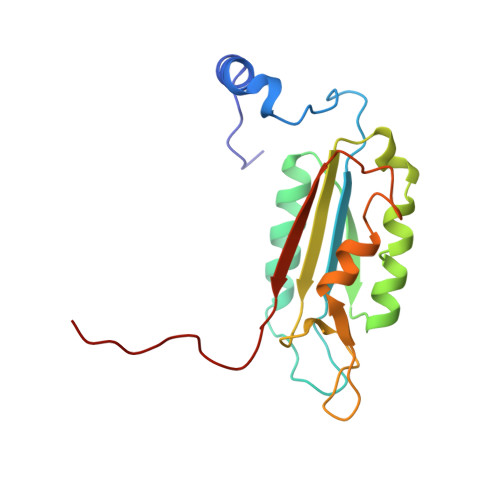
Based on the X-ray structure of the complex of Ac-Tyr-Val-Ala-Asp-H (L-709049) and interleukin-1 beta converting enzyme (ICE), we synthesized compounds which were derived from 2-NapCO-Val-Pro-Asp-CH2OPh (1) to obtain a potent inhibitor in the cell assay. Among these compounds, (3S)-N-methanesulfonyl-3-[[1-[N-(2-naphthoyl)-L-valyl]-L-prolyl]amino]- 4-oxobutanamide (27c) showed high potency not only in the enzyme assay but also cell assay with IC50 values of 38 nM and 0.23 microM, respectively. Compound 27c, with a c log P value of 1.76, had a more hydrophilic character compared with 1. Compound 27c also dose dependently inhibited LPS-primed ATP-induced IL-1 beta release in mice. The crystal structure of the complex of compound 27c and ICE revealed that compound 27c had further interactions with ICE in the naphthoyl group at the P4 position and in the methyl group of the methanesulfonamidecarbonyl group at the P1 position, compared with L-709049. To our knowledge, compound 27c is the first example that shows a strong inhibitory activity without the carboxyl group at the P1 position.
Organizational Affiliation:
Institute for Drug Discovery Research, Yamanouchi Pharmaceutical Co. Ltd., Ibaraki, Japan.