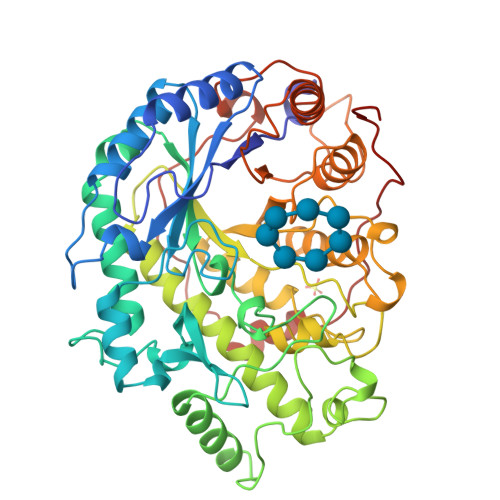
Crystal structure of recombinant soybean beta-amylase complexed with beta-cyclodextrin.
Adachi, M., Mikami, B., Katsube, T., Utsumi, S.(1998) J Biological Chem 273: 19859-19865
- PubMed: 9677422
- DOI: https://doi.org/10.1074/jbc.273.31.19859
- Primary Citation of Related Structures:
1BFN - PubMed Abstract:
In order to study the interaction of soybean beta-amylase with substrate, we solved the crystal structure of beta-cyclodextrin-enzyme complex and compared it with that of alpha-cyclodextrin-enzyme complex. The enzyme was expressed in Escherichia coli at a high level as a soluble and catalytically active protein. The purified recombinant enzyme had properties nearly identical to those of native soybean beta-amylase and formed the same crystals as the native enzyme. The crystal structure of recombinant enzyme complexed with beta-cyclodextrin was refined at 2. 07-A resolution with a final crystallographic R value of 15.8% (Rfree = 21.1%). The root mean square deviation in the position of C-alpha atoms between this recombinant enzyme and the native enzyme was 0.22 A. These results indicate that the expression system established here is suitable for studying structure-function relationships of beta-amylase. The conformation of the bound beta-cyclodextrin takes an ellipsoid shape in contrast to the circular shape of the bound alpha-cyclodextrin. The cyclodextrins shared mainly two glucose binding sites, 3 and 4. The glucose residue 4 was slightly shifted from the maltose binding site. This suggests that the binding site of the cyclodextrins is important for its holding of a cleaved substrate, which enables the multiple attack mechanism of beta-amylase.
Organizational Affiliation:
Research Institute for Food Science, Kyoto University, Uji Kyoto 611-0011, Japan.