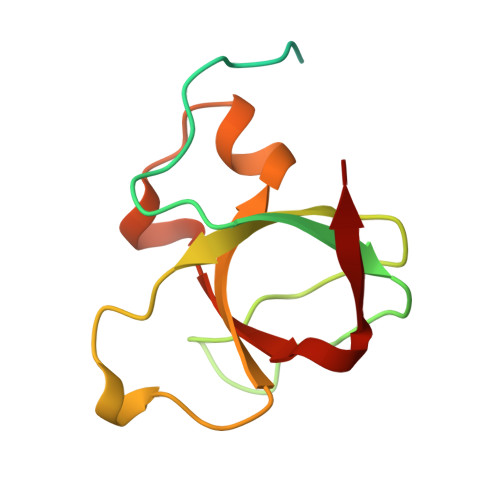
Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation.
Owen, D.J., Wigge, P., Vallis, Y., Moore, J.D., Evans, P.R., McMahon, H.T.(1998) EMBO J 17: 5273-5285
- PubMed: 9736607
- DOI: https://doi.org/10.1093/emboj/17.18.5273
- Primary Citation of Related Structures:
1BB9 - PubMed Abstract:
The amphiphysins are brain-enriched proteins, implicated in clathrin-mediated endocytosis, that interact with dynamin through their SH3 domains. To elucidate the nature of this interaction, we have solved the crystal structure of the amphiphysin-2 (Amph2) SH3 domain to 2.2 A. The structure possesses several notable features, including an extensive patch of negative electrostatic potential covering a large portion of its dynamin binding site. This patch accounts for the specific requirement of amphiphysin for two arginines in the proline-rich binding motif to which it binds on dynamin. We demonstrate that the interaction of dynamin with amphiphysin SH3 domains, unlike that with SH3 domains of Grb2 or spectrin, prevents dynamin self-assembly into rings. Deletion of a unique insert in the n-Src loop of Amph2 SH3, a loop adjacent to the dynamin binding site, significantly reduces this effect. Conversely, replacing the n-Src loop of the N-terminal SH3 domain of Grb2 with that of Amph2 causes it to favour dynamin ring disassembly. Transferrin uptake assays show that shortening the n-Src loop of Amph2 SH3 reduces the ability of this domain to inhibit endocytosis in vivo. Our data suggest that amphiphysin SH3 domains are important regulators of the multimerization cycle of dynamin in endocytosis.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK.