Structural characterization of activation 'intermediate 2' on the pathway to human gastricsin.
Khan, A.R., Cherney, M.M., Tarasova, N.I., James, M.N.(1997) Nat Struct Biol 4: 1010-1015
- PubMed: 9406551
- DOI: https://doi.org/10.1038/nsb1297-1010
- Primary Citation of Related Structures:
1AVF - PubMed Abstract:
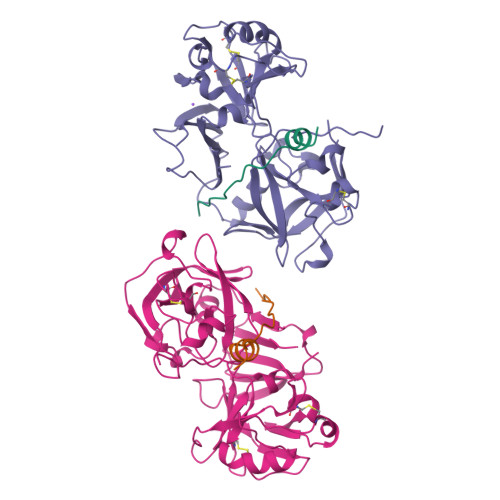
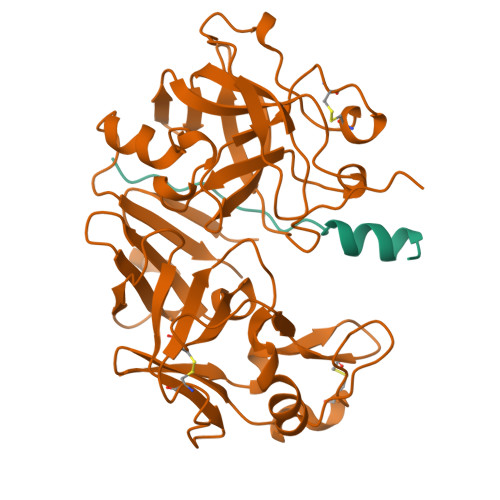
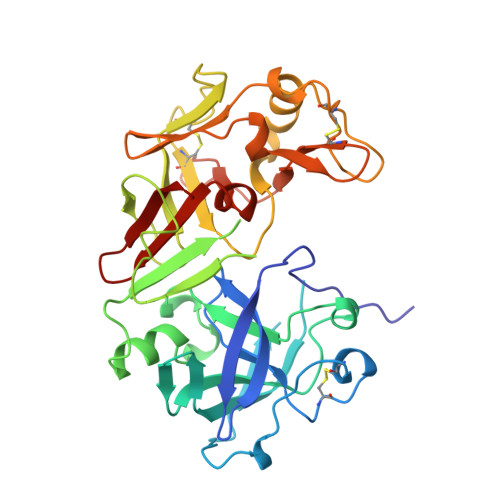
The crystal structure of an activation intermediate of human gastricsin has been determined at 2.4 A resolution. The human digestive enzyme gastricsin (pepsin C) is an aspartic proteinase that is synthesized as the inactive precursor (zymogen) progastricsin (pepsinogen C or hPGC). In the zymogen, a positively-charged N-terminal prosegment of 43 residues (Ala 1p-Leu 43p; the suffix 'p' refers to the prosegment) sterically prevents the approach of a substrate to the active site. Zymogen conversion occurs in an autocatalytic and stepwise fashion at low pH through the formation of intermediates. The structure of the non-covalent complex of a partially-cleaved peptide of the prosegment (Ala 1p-Phe 26p) with mature gastricsin (Ser 1-Ala 329) suggests an activation pathway that may be common to all gastric aspartic proteinases.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, Canada.