The crystal structure of bovine bile salt activated lipase: insights into the bile salt activation mechanism.
Wang, X., Wang, C.S., Tang, J., Dyda, F., Zhang, X.C.(1997) Structure 5: 1209-1218
- PubMed: 9331420
- DOI: https://doi.org/10.1016/s0969-2126(97)00271-2
- Primary Citation of Related Structures:
1AKN, 1AQL - PubMed Abstract:
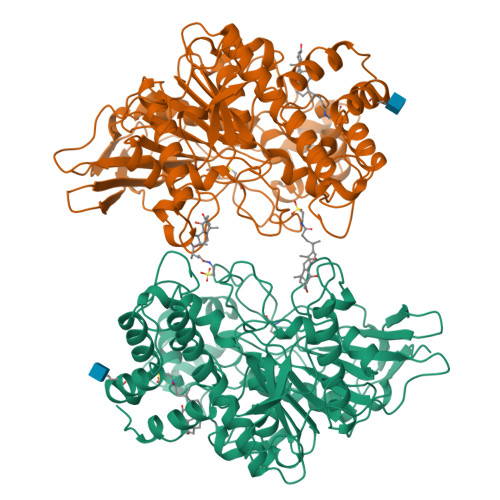
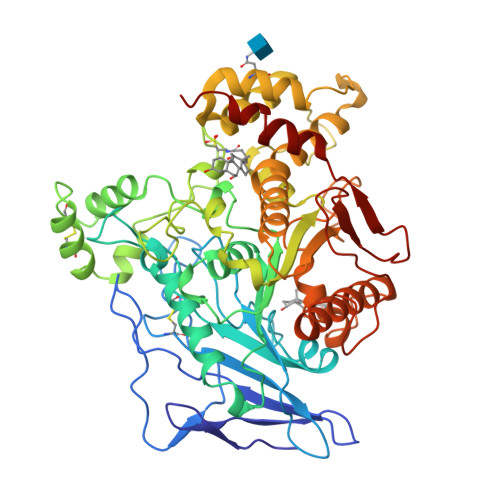
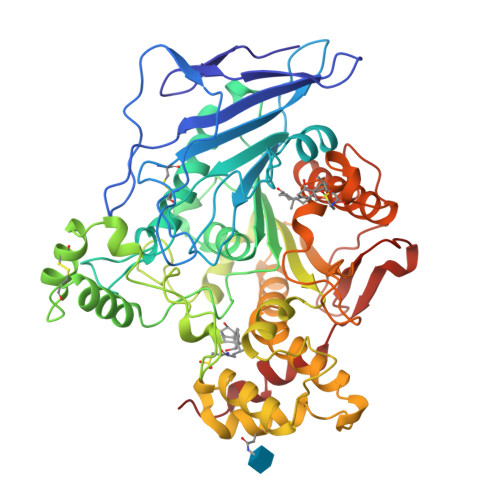
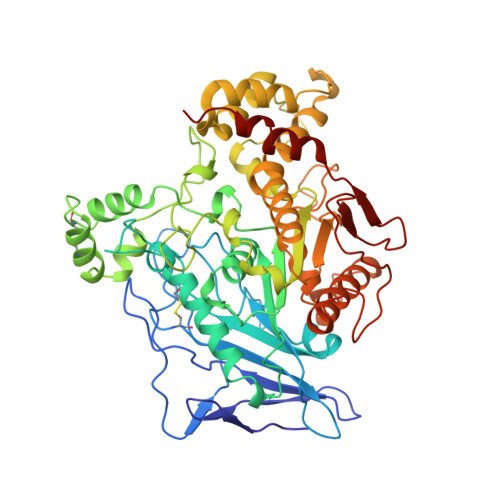
The intestinally located pancreatic enzyme, bile salt activated lipase (BAL), possesses unique activities for digesting different kinds of lipids. It also differs from other lipases in a requirement of bile salts for activity. A structure-based explanation for these unique properties has not been reached so far due to the absence of a three-dimensional structure. The crystal structures of bovine BAL and its complex with taurocholate have been determined at 2.8 A resolution. The overall structure of BAL belongs to the alpha/beta hydrolase fold family. Two bile salt binding sites were found in each BAL molecule within the BAL-taurocholate complex structure. One of these sites is located close to a hairpin loop near the active site. Upon the binding of taurocholate, this loop becomes less mobile and assumes a different conformation. The other bile salt binding site is located remote from the active site. In both structures, BAL forms similar dimers with the active sites facing each other. Bile salts activate BAL by binding to a relatively short ten-residue loop near the active site, and stabilize the loop in an open conformation. Presumably, this conformational change leads to the formation of the substrate-binding site, as suggested from kinetic data. The BAL dimer observed in the crystal structure may also play a functional role under physiological conditions.
Organizational Affiliation:
Crystallography Program, Oklahoma Medical Research Foundation, Oklahoma City 73104, USA.