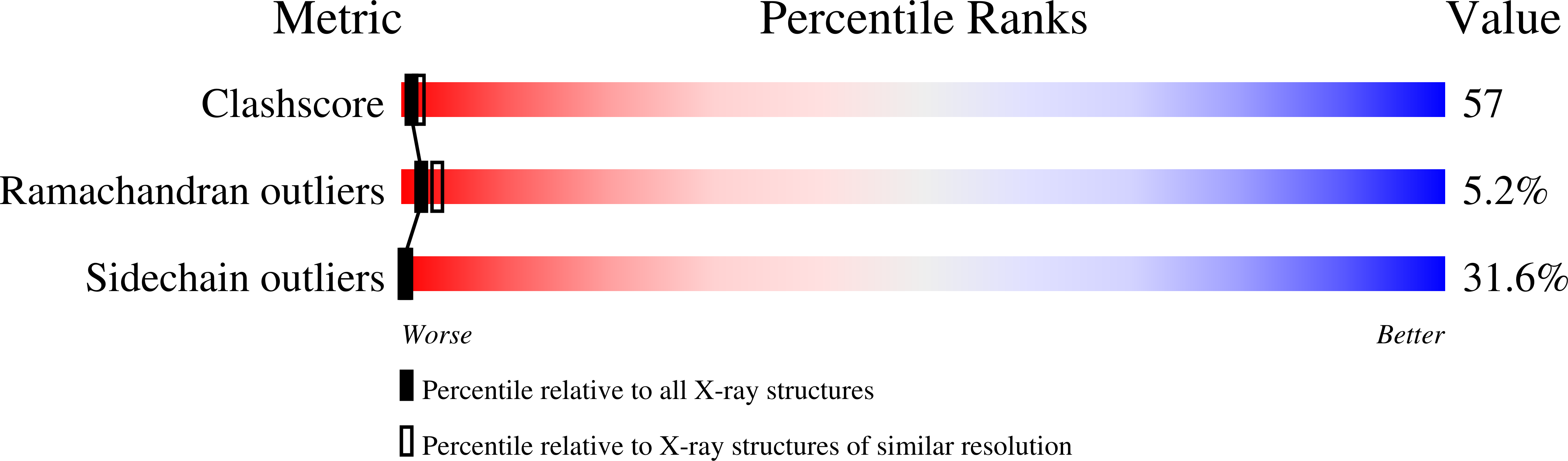Structural studies of the binding of the anti-ulcer drug sucrose octasulfate to acidic fibroblast growth factor.
Zhu, X., Hsu, B.T., Rees, D.C.(1993) Structure 1: 27-34
- PubMed: 7520817
- DOI: https://doi.org/10.1016/0969-2126(93)90006-3
- Primary Citation of Related Structures:
1AFC - PubMed Abstract:
The anti-ulcer drug sucrose octasulfate (SOS) binds to fibroblast growth factors (FGFs), proteins which stimulate the growth and differentiation of several cell types, including stomach epithelial cells. It is believed that SOS stabilizes FGFs against acid denaturation in the stomach, thus enhancing their ability to stimulate healing of ulcerated tissue. SOS binds to the same site on FGF as heparin and other proteoglycans; in vivo, FGF must bind to cell-surface proteoglycans or to heparin before it can interact with FGF receptors and stimulate growth. The details of this process are not understood. We report the crystal structure of a 1:1 complex between acidic FGF (aFGF) and SOS at 2.7 A resolution. SOS binds to a positively charged region of aFGF, largely composed of residues 112-127, and makes contacts primarily with Lys112, Arg116, Lys118, and Arg122. This region is also important in binding heparin. The overall conformation of aFGF is not changed by binding SOS, although the positions of some side chains in the binding site shift by as much as 6 A. The SOS-FGF crystal structure is consistent with the model that SOS stabilizes FGF by neutralizing several positively charged residues that would destabilize the native structure by electrostatic repulsion. On the basis of this structure, we provide a model for the complex of heparin with an FGF dimer. Such interactions may facilitate FGF receptor dimerization, which may be important in receptor signaling.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena 91125.