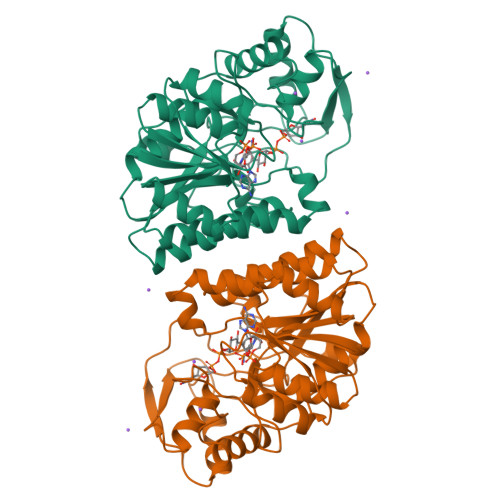
Dramatic differences in the binding of UDP-galactose and UDP-glucose to UDP-galactose 4-epimerase from Escherichia coli.
Thoden, J.B., Holden, H.M.(1998) Biochemistry 37: 11469-11477
- PubMed: 9708982
- DOI: https://doi.org/10.1021/bi9808969
- Primary Citation of Related Structures:
1A9Y, 1A9Z - PubMed Abstract:
UDP-galactose 4-epimerase catalyzes the interconversion of UDP-galactose and UDP-glucose during normal galactose metabolism. Within recent years the enzyme from Escherichia coli has been studied extensively by both biochemical and X-ray crystallographic techniques. One of several key features in the catalytic mechanism of the enzyme involves the putative rotation of a 4'-ketopyranose intermediate within the active site region. The mode of binding of UDP-glucose to epimerase is well understood on the basis of previous high-resolution X-ray crystallographic investigations from this laboratory with an enzyme/NADH/UDP-glucose abortive complex. Attempts to prepare an enzyme/NADH/UDP-galactose abortive complex always failed, however, in that UDP-glucose rather than UDP-galactose was observed binding in the active site. In an effort to prepare an abortive complex with UDP-galactose, a site-directed mutant protein was constructed in which Ser 124 and Tyr 149, known to play critical roles in catalysis, were substituted with alanine and phenylalanine residues, respectively. With this double mutant it was possible to crystallize and solve the three-dimensional structures of reduced epimerase in the presence of UDP-glucose or UDP-galactose to high resolution. This study represents the first direct observation of UDP-galactose binding to epimerase and lends strong structural support for a catalytic mechanism in which there is free rotation of a 4'-ketopyranose intermediate within the active site cleft of the enzyme.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin-Madison 53705, USA.