The effects of nature-inspired amino acid substitutions on structural and biochemical properties of the E. coli L-asparaginase EcAIII.
Janicki, M., Sciuk, A., Zielezinski, A., Ruszkowski, M., Ludwikow, A., Karlowski, W.M., Jaskolski, M., Loch, J.I.(2023) Protein Sci 32: e4647-e4647
- PubMed: 37095066
- DOI: https://doi.org/10.1002/pro.4647
- Primary Citation of Related Structures:
8BI3, 8BKF, 8BP9, 8BQO, 8C0I, 8C23 - PubMed Abstract:
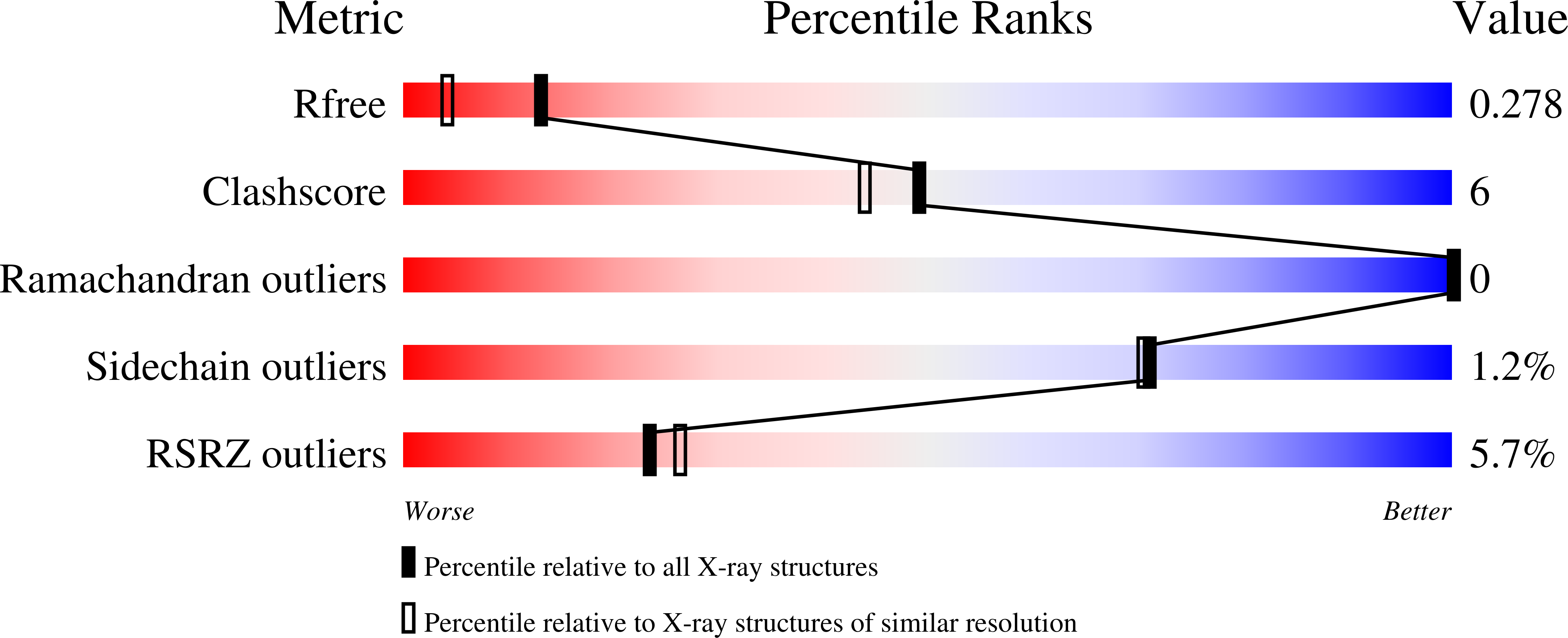
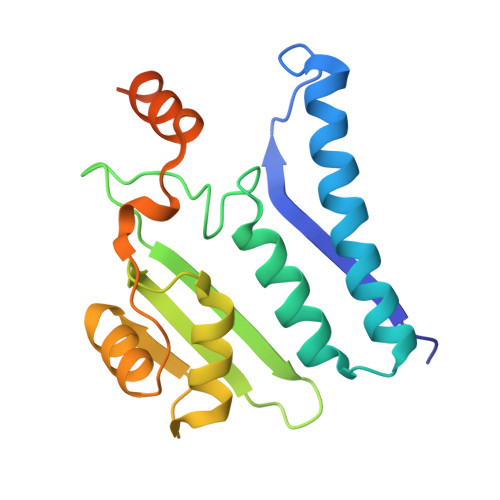
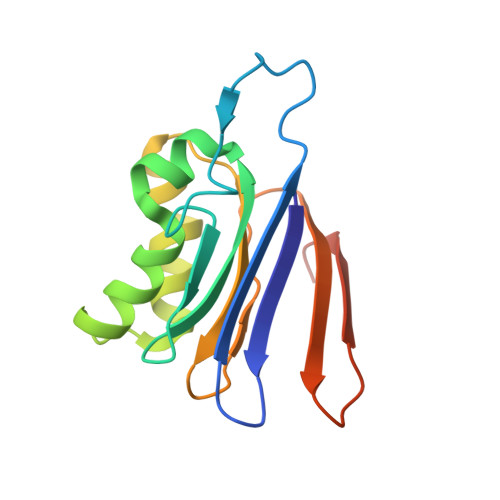
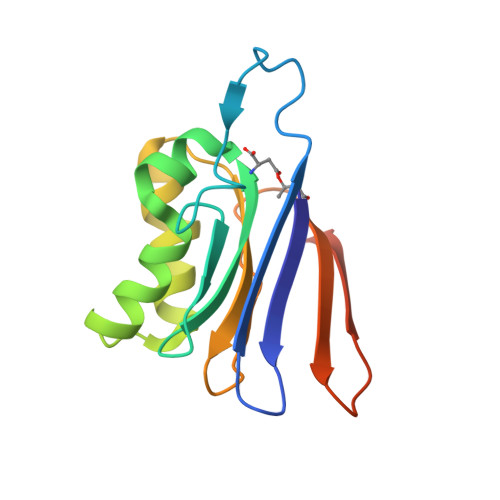
The Escherichia coli enzyme EcAIII catalyzes the hydrolysis of L-Asn to L-Asp and ammonia. Using a nature-inspired mutagenesis approach, we designed and produced five new EcAIII variants (M200I, M200L, M200K, M200T, M200W). The modified proteins were characterized by spectroscopic and crystallographic methods. All new variants were enzymatically active, confirming that the applied mutagenesis procedure has been successful. The determined crystal structures revealed new conformational states of the EcAIII molecule carrying the M200W mutation and allowed a high-resolution observation of an acyl-enzyme intermediate with the M200L mutant. In addition, we performed structure prediction, substrate docking, and molecular dynamics simulations for 25 selected bacterial orthologs of EcAIII, to gain insights into how mutations at the M200 residue affect the active site and substrate binding mode. This comprehensive strategy, including both experimental and computational methods, can be used to guide further enzyme engineering and can be applied to the study of other proteins of medicinal or biotechnological importance.
Organizational Affiliation:
Department of Biotechnology, Institute of Molecular Biology and Biotechnology, Faculty of Biology, Adam Mickiewicz University, Poznań, Poland.