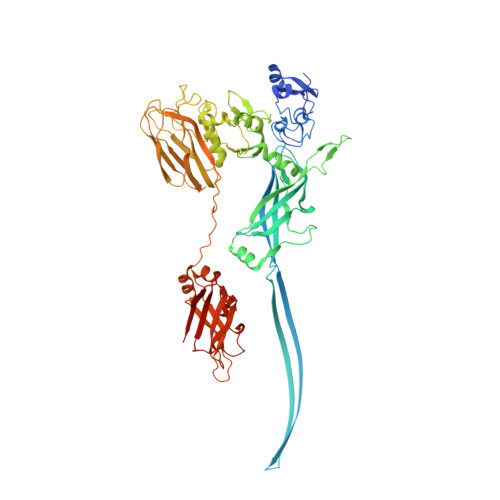
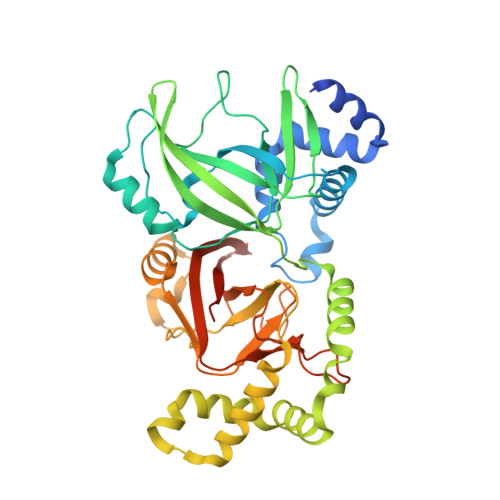
Cryo-EM structures of the translocational binary toxin complex CDTa-bound CDTb-pore from Clostridioides difficile.
Kawamoto, A., Yamada, T., Yoshida, T., Sato, Y., Kato, T., Tsuge, H.(2022) Nat Commun 13: 6119-6119
- PubMed: 36253419
- DOI: https://doi.org/10.1038/s41467-022-33888-4
- Primary Citation of Related Structures:
7VNJ, 7VNN, 7YVQ, 7YVS - PubMed Abstract:
Some bacteria express a binary toxin translocation system, consisting of an enzymatic subunit and translocation pore, that delivers enzymes into host cells through endocytosis. The most clinically important bacterium with such a system is Clostridioides difficile (formerly Clostridium). The CDTa and CDTb proteins from its system represent important therapeutic targets. CDTb has been proposed to be a di-heptamer, but its physiological heptameric structure has not yet been reported. Here, we report the cryo-EM structure of CDTa bound to the CDTb-pore, which reveals that CDTa binding induces partial unfolding and tilting of the first CDTa α-helix. In the CDTb-pore, an NSS-loop exists in 'in' and 'out' conformations, suggesting its involvement in substrate translocation. Finally, 3D variability analysis revealed CDTa movements from a folded to an unfolded state. These dynamic structural information provide insights into drug design against hypervirulent C. difficile strains.
Organizational Affiliation:
Institute for Protein Research, Osaka University, Suita, Osaka, 565-0871, Japan.