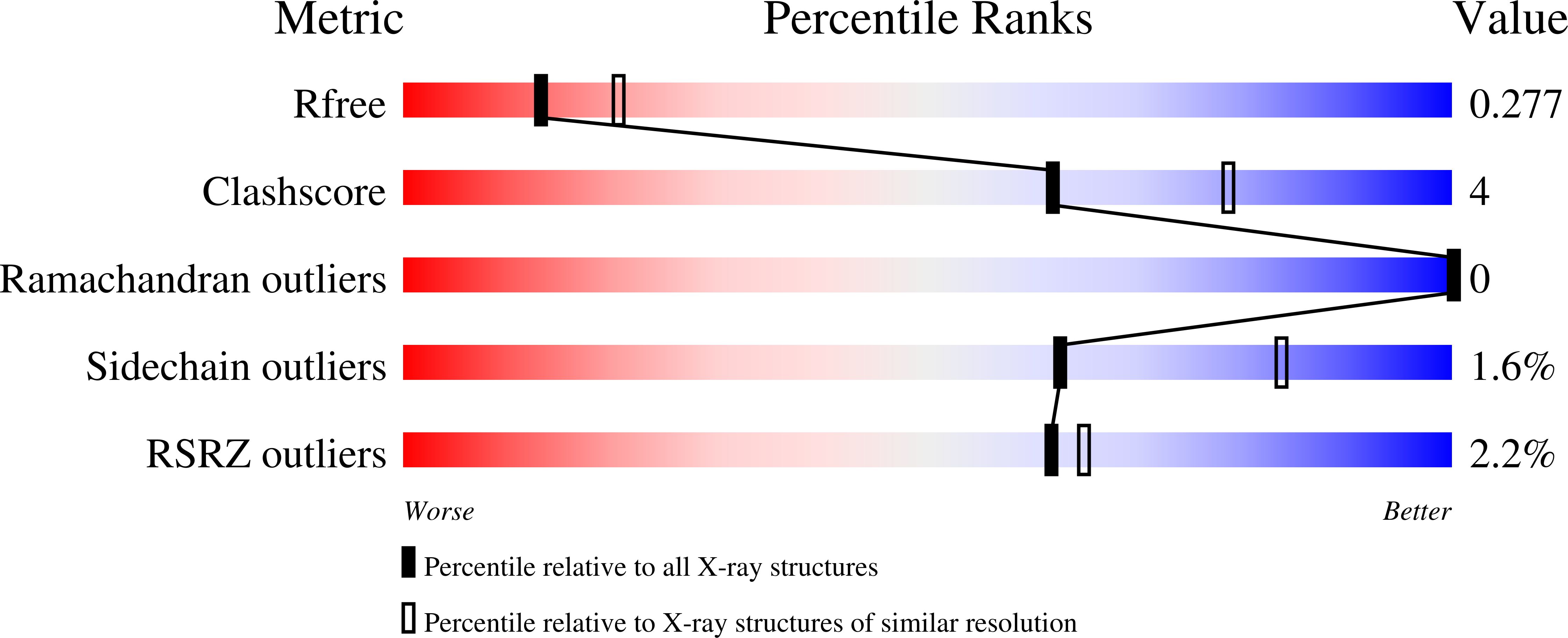
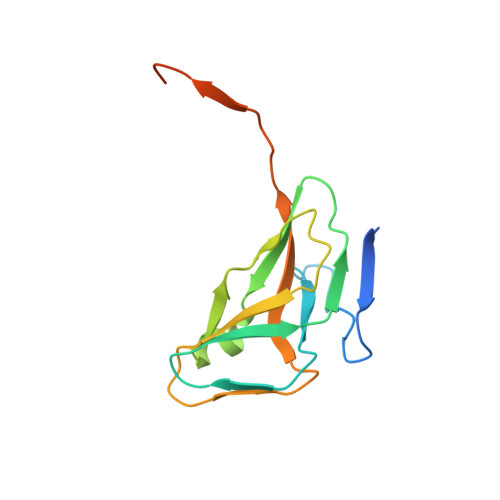
Structures of vaccinia virus dUTPase and its nucleotide complexes.
Samal, A., Schormann, N., Cook, W.J., Delucas, L.J., Chattopadhyay, D.(2007) Acta Crystallogr D Biol Crystallogr 63: 571-580
- PubMed: 17452782
- DOI: https://doi.org/10.1107/S0907444907007871
- Primary Citation of Related Structures:
2OKB, 2OKD, 2OKE, 2OL0, 2OL1 - PubMed Abstract:
Deoxyuridine triphosphate nucleotidohydrolase (dUTPase) catalyzes the hydrolysis of dUTP to dUMP and pyrophosphate in the presence of Mg(2+) ions. The enzyme plays multiple cellular roles by maintaining a low dUTP:dTTP ratio and by synthesizing the substrate for thymidylate synthase in the biosynthesis of dTTP. Although dUTPase is an essential enzyme and has been established as a valid target for drug design, the high degree of homology of vaccinia virus dUTPase to the human enzyme makes the identification of selective inhibitors difficult. The crystal structure of vaccinia virus dUTPase has been solved and the active site has been mapped by crystallographic analysis of the apo enzyme and of complexes with the substrate-analog dUMPNPP, with the product dUMP and with dUDP, which acts as an inhibitor. Analyses of these structures reveal subtle differences between the viral and human enzymes. In particular, the much larger size of the central channel at the trimer interface suggests new possibilities for structure-based drug design. Vaccinia virus is a prototype of the poxviruses.
Organizational Affiliation:
Center for Biophysical Sciences and Engineering, University of Alabama at Birmingham, Birmingham, AL 35294, USA.