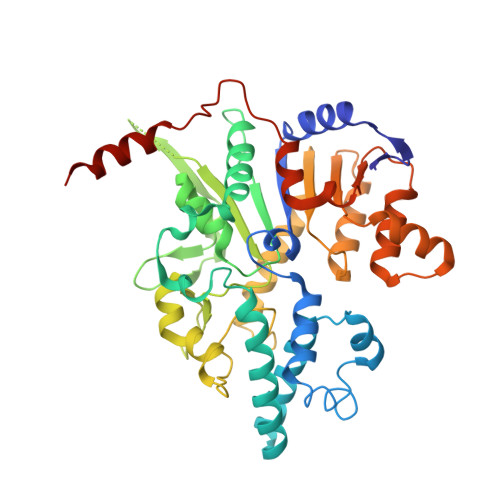
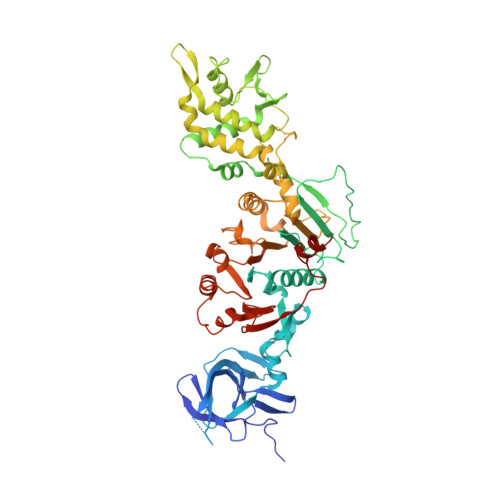
Assembly-mediated activation of the SIR2-HerA supramolecular complex for anti-phage defense.
Shen, Z., Lin, Q., Yang, X.Y., Fosuah, E., Fu, T.M.(2023) Mol Cell 83: 4586-4599.e5
- PubMed: 38096827
- DOI: https://doi.org/10.1016/j.molcel.2023.11.007
- Primary Citation of Related Structures:
8SU9, 8SUB, 8SUW, 8SXX, 8UAE, 8UAF - PubMed Abstract:
SIR2-HerA, a bacterial two-protein anti-phage defense system, induces bacterial death by depleting NAD + upon phage infection. Biochemical reconstitution of SIR2, HerA, and the SIR2-HerA complex reveals a dynamic assembly process. Unlike other ATPases, HerA can form various oligomers, ranging from dimers to nonamers. When assembled with SIR2, HerA forms a hexamer and converts SIR2 from a nuclease to an NAD + hydrolase, representing an unexpected regulatory mechanism mediated by protein assembly. Furthermore, high concentrations of ATP can inhibit NAD + hydrolysis by the SIR2-HerA complex. Cryo-EM structures of the SIR2-HerA complex reveal a giant supramolecular assembly up to 1 MDa, with SIR2 as a dodecamer and HerA as a hexamer, crucial for anti-phage defense. Unexpectedly, the HerA hexamer resembles a spiral staircase and exhibits helicase activities toward dual-forked DNA. Together, we reveal the supramolecular assembly of SIR2-HerA as a unique mechanism for switching enzymatic activities and bolstering anti-phage defense strategies.
Organizational Affiliation:
Department of Biological Chemistry and Pharmacology, The Center for RNA Biology, The Ohio State University, Columbus, OH 43210, USA; The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA.