Structural basis for the substrate specificity of an S-formylglutathione hydrolase derived from Variovorax sp. PAMC 28711.
Hwang, J., Kim, B., Lee, M.J., Nam, Y., Youn, U.J., Lee, C.S., Oh, T.J., Park, H.H., Do, H., Lee, J.H.(2022) Biochem Biophys Res Commun 629: 159-164
- PubMed: 36122453
- DOI: https://doi.org/10.1016/j.bbrc.2022.09.008
- Primary Citation of Related Structures:
7YVT - PubMed Abstract:
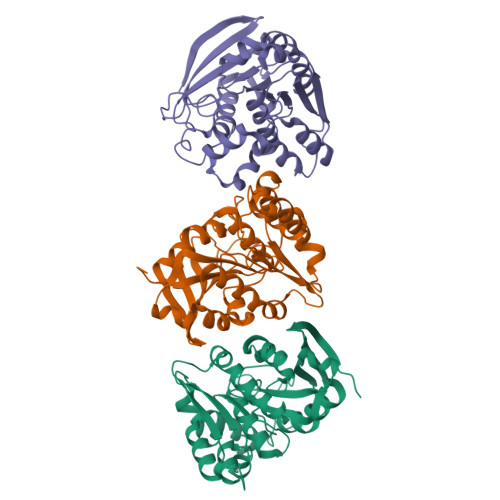
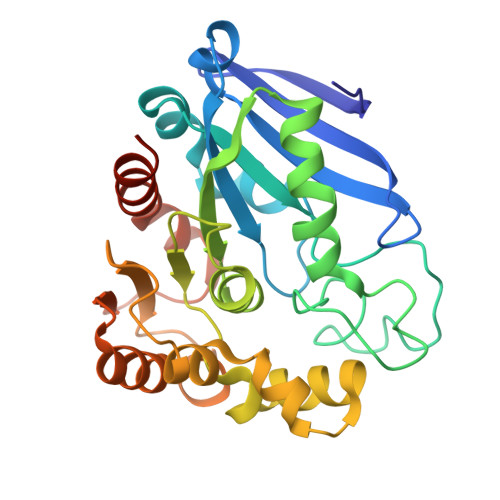
S-Formylglutathione hydrolase was originally known to catalyze the hydrolysis of S-formylglutathione to formate and glutathione. However, this enzyme has a broader esterase activity toward substrates containing thioester and ester bonds. In a previous study, we identified a new S-formylglutathione hydrolase (VaSFGH) gene in the Antarctic bacterium Variovorax sp. PAMC 28711, and recombinant VaSFGH protein was purified and characterized. Previous enzyme activity assays showed that VaSFGH has high activity, especially toward short-chain p-nitrophenyl esters (C2-C4). In this study, we determined the crystal structure of substrate-free VaSFGH at a resolution of 2.38 Å. In addition, p-nitrophenyl ester-bound VaSFGH structure models were generated by molecular docking simulations to obtain structural evidence of its substrate specificity. Comparative structural analysis of the apo-form and p-nitrophenyl ester-bound VaSFGH model structures revealed that large substrates could not bind inside the hydrophobic substrate-binding pocket because of the intrinsically static and relatively small substrate-binding pocket size of VaSFGH. This study provides useful information for further protein engineering of SFGHs for industrial use.
Organizational Affiliation:
Research Unit of Cryogenic Novel Material, Korea Polar Research Institute, Incheon, 21990, Republic of Korea; Department of Polar Sciences, University of Science and Technology, Incheon, 21990, Republic of Korea.