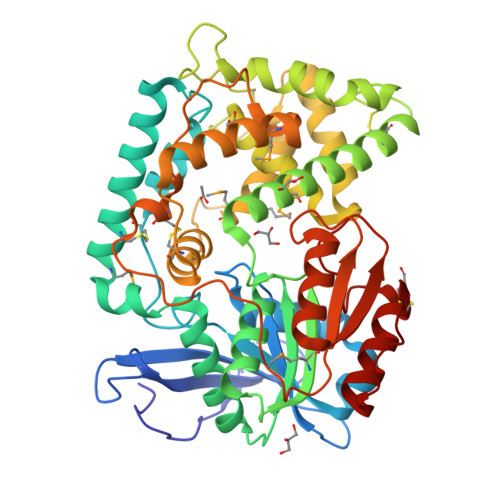
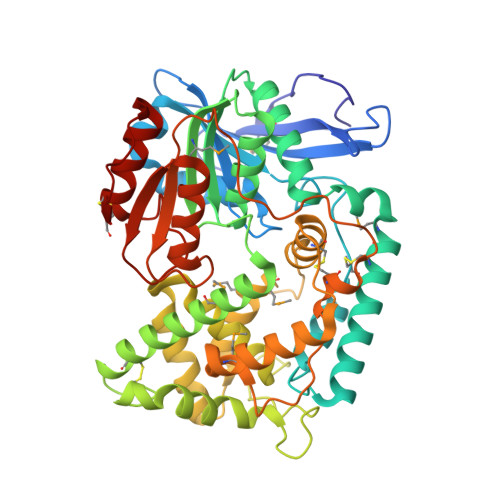
2.1 angstrom crystal structure of the Mycobacterium tuberculosis serine hydrolase, Hip1, in its anhydro-form (Anhydrohip1).
Brooks, C.L., Ostrov, D.A., Schumann, N.C., Kakkad, S., Li, D., Pena, K., Williams, B.P., Goldfarb, N.E.(2022) Biochem Biophys Res Commun 630: 57-63
- PubMed: 36148729
- DOI: https://doi.org/10.1016/j.bbrc.2022.09.021
- Primary Citation of Related Structures:
7SFM, 8E5W - PubMed Abstract:
The 2.6 Å crystal structure of the apo form of Hip1 (hydrolase important for pathogenesis) has been previously reported. However, very little is known about the active site architecture of this M. tuberculosis (Mtb), serine hydrolase drug target. To begin mapping the active site of Hip1, we cocrystallized Hip1 with the irreversible serine protease inhibitor, 4-(2-aminoethyl)-benzenesulfonylfluoride (AEBSF). We chose AEBSF for cocrystallization with Hip1 since the similar inhibitor, phenylmethylsulfonyl fluoride (PMSF), interestingly exhibited no activity against Hip1. We obtained crystals that diffracted to 2.1 Å but to our bewilderment, we did not observe any electron density for the inhibitor in the omit map for the Hip1-AEBSF complex. Rather, in the active site, dehydroalanine (dAla) was found to occupy the expected position of the catalytic Ser228, thus yielding anhydrohip1. Here we present a comparative analysis of the crystal structures of anhydrohip1 and Hip1 and provide a mechanism for the conversion of the enzyme to the anhydro-form through reaction with AEBSF. With the aid of molecular docking, we propose an explanation for the differential inhibition of Hip1 by AEBSF and PMSF. We also present a preliminary definition of the S1 and S2 pockets of the protease's active site and propose a mechanism for a ligand-induced conformational change within the S2 pocket. Finally, we expand upon the previous demarcation of the putative lipid binding pocket in the α-domain of the enzyme. We believe that this detailed analysis of the structures of anhydrohip1 and Hip1 provides valuable information useful for the structure-based drug design of novel Hip1-directed Mtb therapeutics.
Organizational Affiliation:
Department of Chemistry and Biochemistry, California State University, Fresno, CA, USA.