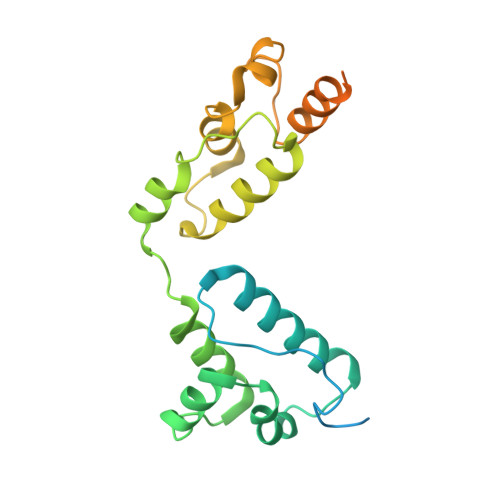
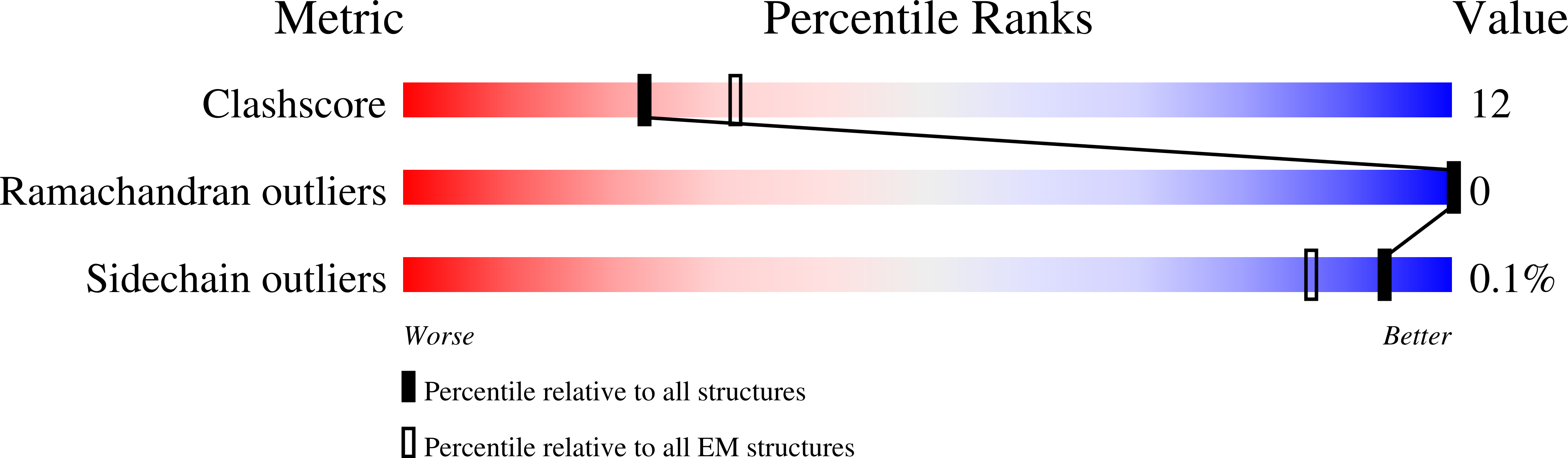Structures of virus-like capsids formed by the Drosophila neuronal Arc proteins.
Erlendsson, S., Morado, D.R., Cullen, H.B., Feschotte, C., Shepherd, J.D., Briggs, J.A.G.(2020) Nat Neurosci 23: 172-175
- PubMed: 31907439
- DOI: https://doi.org/10.1038/s41593-019-0569-y
- Primary Citation of Related Structures:
6TAP, 6TAQ, 6TAR, 6TAS, 6TAT, 6TAU - PubMed Abstract:
Arc, a neuronal gene that is critical for synaptic plasticity, originated through the domestication of retrotransposon Gag genes and mediates intercellular messenger RNA transfer. We report high-resolution structures of retrovirus-like capsids formed by Drosophila dArc1 and dArc2 that have surface spikes and putative internal RNA-binding domains. These data demonstrate that virus-like capsid-forming properties of Arc are evolutionarily conserved and provide a structural basis for understanding their function in intercellular communication.
Organizational Affiliation:
Structural Studies Division, MRC Laboratory of Molecular Biology, Cambridge, UK.