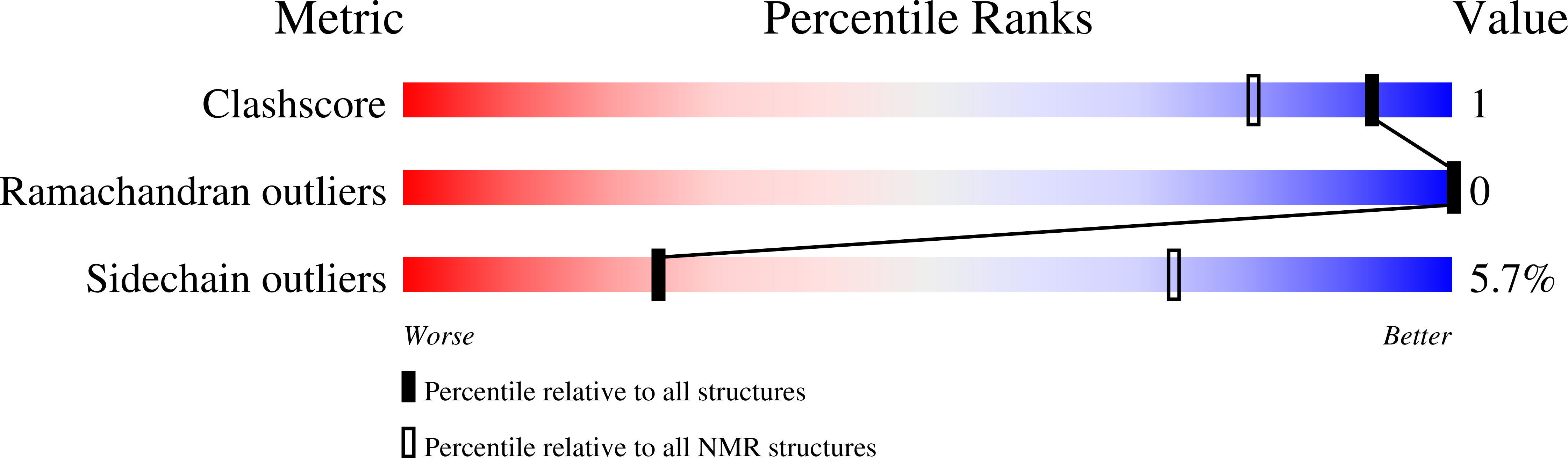The host-defense peptide piscidin P1 reorganizes lipid domains in membranes and decreases activation energies in mechanosensitive ion channels.
Comert, F., Greenwood, A., Maramba, J., Acevedo, R., Lucas, L., Kulasinghe, T., Cairns, L.S., Wen, Y., Fu, R., Hammer, J., Blazyk, J., Sukharev, S., Cotten, M.L., Mihailescu, M.(2019) J Biological Chem 294: 18557-18570
- PubMed: 31619519
- DOI: https://doi.org/10.1074/jbc.RA119.010232
- Primary Citation of Related Structures:
6PEZ, 6PF0 - PubMed Abstract:
The host-defense peptide (HDP) piscidin 1 (P1), isolated from the mast cells of striped bass, has potent activities against bacteria, viruses, fungi, and cancer cells and can also modulate the activity of membrane receptors. Given its broad pharmacological potential, here we used several approaches to better understand its interactions with multicomponent bilayers representing models of bacterial (phosphatidylethanolamine (PE)/phosphatidylglycerol) and mammalian (phosphatidylcholine/cholesterol (PC/Chol)) membranes. Using solid-state NMR, we solved the structure of P1 bound to PC/Chol and compared it with that of P3, a less potent homolog. The comparison disclosed that although both peptides are interfacially bound and α-helical, they differ in bilayer orientations and depths of insertion, and these differences depend on bilayer composition. Although Chol is thought to make mammalian membranes less susceptible to HDP-mediated destabilization, we found that Chol does not affect the permeabilization effects of P1. X-ray diffraction experiments revealed that both piscidins produce a demixing effect in PC/Chol membranes by increasing the fraction of the Chol-depleted phase. Furthermore, P1 increased the temperature required for the lamellar-to-hexagonal phase transition in PE bilayers, suggesting that it imposes positive membrane curvature. Patch-clamp measurements on the inner Escherichia coli membrane showed that P1 and P3, at concentrations sufficient for antimicrobial activity, substantially decrease the activating tension for bacterial mechanosensitive channels. This indicated that piscidins can cause lipid redistribution and restructuring in the microenvironment near proteins. We conclude that the mechanism of piscidin's antimicrobial activity extends beyond simple membrane destabilization, helping to rationalize its broader spectrum of pharmacological effects.
Organizational Affiliation:
Institute for Bioscience and Biotechnology Research, Rockville, Maryland 20850.