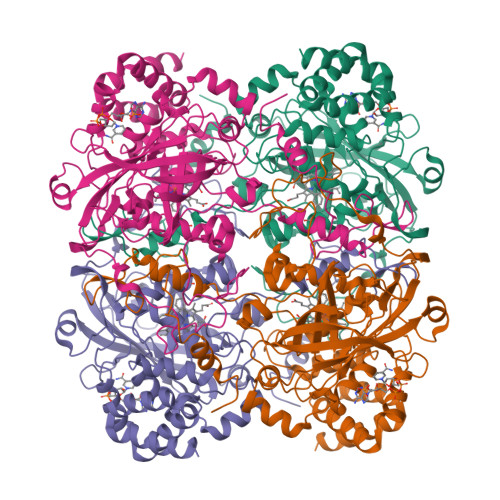
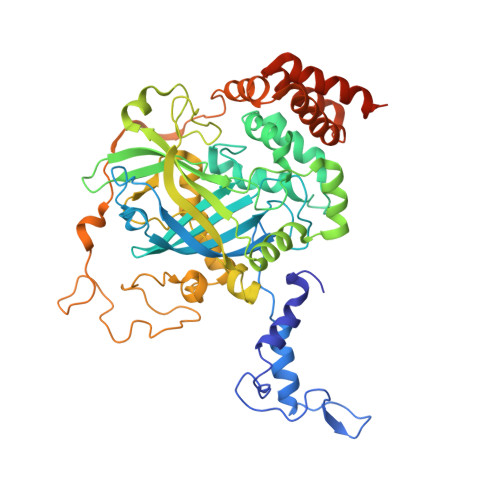
A new cryo-EM system for electron 3D crystallography by eEFD.
Yonekura, K., Ishikawa, T., Maki-Yonekura, S.(2019) J Struct Biol 206: 243-253
- PubMed: 30928615
- DOI: https://doi.org/10.1016/j.jsb.2019.03.009
- Primary Citation of Related Structures:
6JNT, 6JNU - PubMed Abstract:
A new cryo-EM system has been developed and investigated for use in protein electron 3D crystallography. The system provides parallel illumination of a coherent 300 kV electron beam to a sample, filters out energy-loss electrons through the sample with an in-column energy filter, and allows rotational data collection on a fast camera. It also possesses motorized cryo-sample loading and automated liquid-nitrogen filling for cooling of multiple samples. To facilitate its use, we developed GUI programs for efficient operation and accurate structure analysis. Here we report on the performance of the system and first results for thin 3D crystals of the protein complexes, catalase and membrane protein complex ExbBD. Data quality is remarkably improved with this approach, which we name eEFD (electron energy-filtered diffraction of 3D crystals), compared with those collected at 200 kV without energy filtration. Key advances include precise control of the microscope and recordings of lens fluctuations, which the programs process and respond to. We also discuss the merits of higher-energy electrons and filtration of energy-loss electrons in electron 3D crystallography.
Organizational Affiliation:
Biostructural Mechanism Laboratory, RIKEN, SPring-8 Center, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan; Advanced Electron Microscope Development Unit, RIKEN-JEOL Collaboration Center, RIKEN Baton Zone Program, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan. Electronic address: yone@spring8.or.jp.