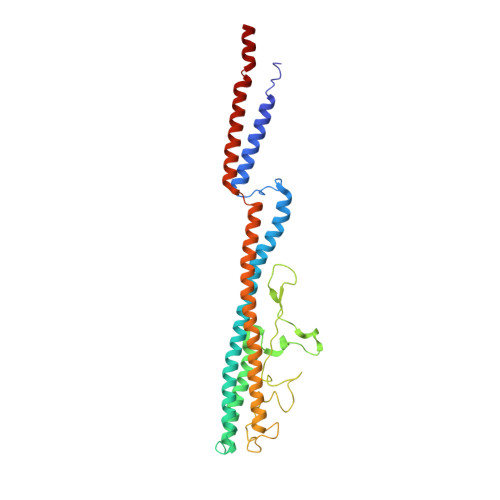
A structural model of flagellar filament switching across multiple bacterial species.
Wang, F., Burrage, A.M., Postel, S., Clark, R.E., Orlova, A., Sundberg, E.J., Kearns, D.B., Egelman, E.H.(2017) Nat Commun 8: 960-960
- PubMed: 29038601
- DOI: https://doi.org/10.1038/s41467-017-01075-5
- Primary Citation of Related Structures:
5WJT, 5WJU, 5WJV, 5WJW, 5WJX, 5WJY, 5WJZ, 5WK5, 5WK6 - PubMed Abstract:
The bacterial flagellar filament has long been studied to understand how a polymer composed of a single protein can switch between different supercoiled states with high cooperativity. Here we present near-atomic resolution cryo-EM structures for flagellar filaments from both Gram-positive Bacillus subtilis and Gram-negative Pseudomonas aeruginosa. Seven mutant flagellar filaments in B. subtilis and two in P. aeruginosa capture two different states of the filament. These reliable atomic models of both states reveal conserved molecular interactions in the interior of the filament among B. subtilis, P. aeruginosa and Salmonella enterica. Using the detailed information about the molecular interactions in two filament states, we successfully predict point mutations that shift the equilibrium between those two states. Further, we observe the dimerization of P. aeruginosa outer domains without any perturbation of the conserved interior of the filament. Our results give new insights into how the flagellin sequence has been "tuned" over evolution.Bacterial flagellar filaments are composed almost entirely of a single protein-flagellin-which can switch between different supercoiled states in a highly cooperative manner. Here the authors present near-atomic resolution cryo-EM structures of nine flagellar filaments, and begin to shed light on the molecular basis of filament switching.
- Department of Biochemistry and Molecular Genetics, University of Virginia School of Medicine, Charlottesville, VA, 22908, USA.
Organizational Affiliation: