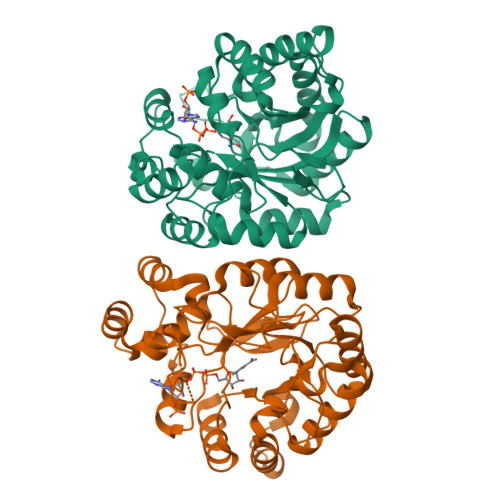
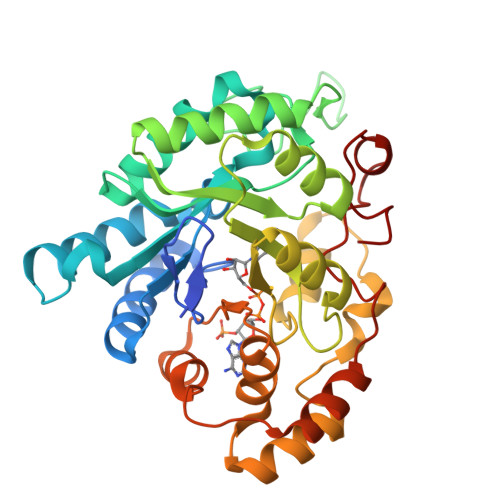
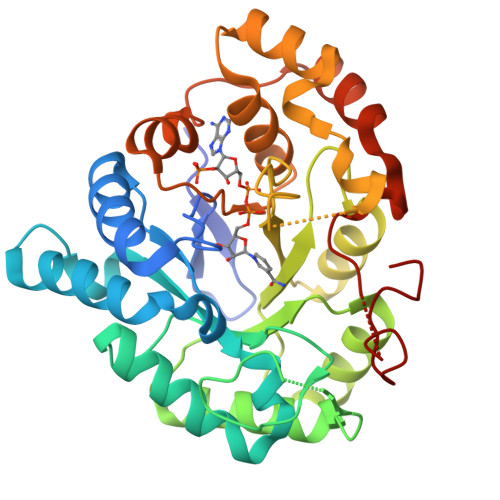
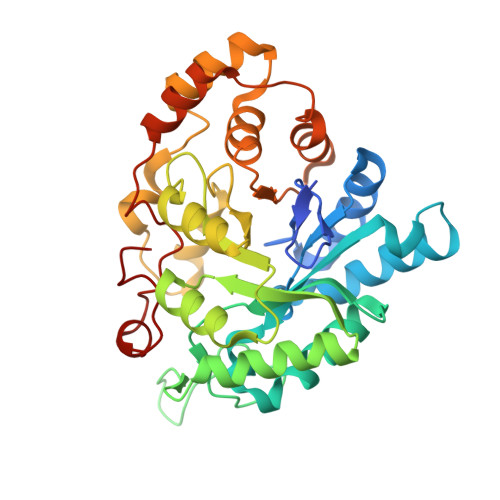
The Coptotermes gestroi aldo-keto reductase: a multipurpose enzyme for biorefinery applications.
Tramontina, R., Franco Cairo, J.P., Liberato, M.V., Mandelli, F., Sousa, A., Santos, S., Rabelo, S.C., Campos, B., Ienczak, J., Ruller, R., Damasio, A.R., Squina, F.M.(2017) Biotechnol Biofuels 10: 4-4
- PubMed: 28053664
- DOI: https://doi.org/10.1186/s13068-016-0688-6
- Primary Citation of Related Structures:
5KET - PubMed Abstract:
In nature, termites can be considered as a model biological system for biofuel research based on their remarkable efficiency for lignocellulosic biomass conversion. Redox enzymes are of interest in second-generation ethanol production because they promote synergic enzymatic activity with classical hydrolases for lignocellulose saccharification and inactivate fermentation inhibitory compounds produced after lignocellulose pretreatment steps. In the present study, the biochemical and structural characteristics of the Coptotermes gestroi aldo-keto reductase ( Cg AKR-1) were comprehensively investigated. Cg AKR-1 displayed major structural differences compared with others AKRs, including the differences in the amino acid composition of the substrate-binding site, providing basis for classification as a founding member of a new AKR subfamily (family AKR1 I). Immunolocalization assays with anti- Cg AKR-1 antibodies resulted in strong fluorescence in the salivary gland, proventriculus, and foregut. Cg AKR-1 supplementation caused a 32% reduction in phenolic aldehydes, such as furfural, which act as fermentation inhibitors of hemicellulosic hydrolysates, and improved ethanol fermentation by the xylose-fermenting yeast Scheffersomyces stipitis by 45%. We observed synergistic enzymatic interactions between Cg AKR-1 and commercial cellulosic cocktail for sugarcane bagasse saccharification, with a maximum synergism degree of 2.17 for sugar release. Our data indicated that additive enzymatic activity could be mediated by reactive oxygen species because Cg AKR-1 could produce hydrogen peroxide. In summary, we identified the founding member of an AKRI subfamily with a potential role in the termite digestome. Cg AKR-1 was found to be a multipurpose enzyme with potential biotechnological applications. The present work provided a basis for the development and application of integrative and multipurpose enzymes in the bioethanol production chain.
Organizational Affiliation:
Laboratório Nacional de Ciência e Tecnologia do Bioetanol (CTBE), Centro Nacional de Pesquisa em Energia e Materiais (CNPEM), Rua Giuseppe Máximo Scolfaro, no 10000 Campinas, SP Brazil ; Programa de Pós Graduação em Biociências e Tecnologia de Produtos Bioativos (BTPB)-Instituto de Biologia-CP 6109, Universidade Estadual de Campinas-UNICAMP, 13083-970 Campinas, SP Brazil.