Crystal structure of the 30 K protein from the silkworm Bombyx mori reveals a new member of the beta-trefoil superfamily
Yang, J.-P., Ma, X.-X., He, Y.-X., Li, W.-F., Kang, Y., Bao, R., Chen, Y., Zhou, C.-Z.(2011) J Struct Biol 175: 97-103
- PubMed: 21514389
- DOI: https://doi.org/10.1016/j.jsb.2011.04.003
- Primary Citation of Related Structures:
3PUB - PubMed Abstract:
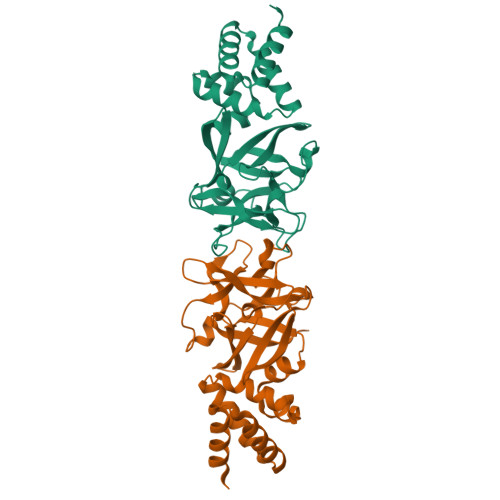
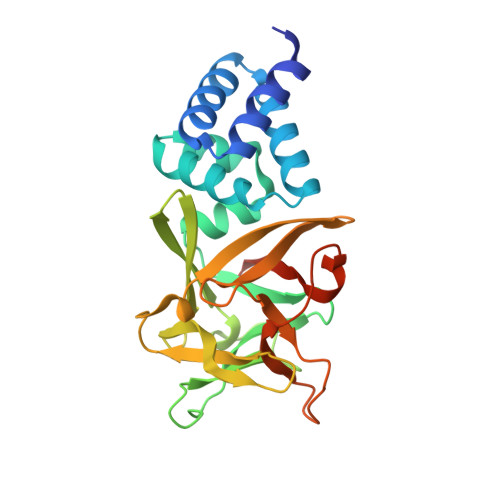
The hemolymph of the fifth instar larvae of the silkworm Bombyx mori contains a group of homologous proteins with a molecular weight of approximately 30 kDa, termed B. mori low molecular weight lipoproteins (Bmlps), which account for about 5% of the total plasma proteins. These so-called "30 K proteins" have been reported to be involved in the innate immune response and transportation of lipid and/or sugar. To elucidate their molecular functions, we determined the crystal structure of a 30 K protein, Bmlp7, at 1.91Å. It has two distinct domains: an all-α N-terminal domain (NTD) and an all-β C-terminal domain (CTD) of the β-trefoil fold. Comparative structural analysis indicates that Bmlp7 represents a new family, adding to the 14 families currently identified, of the β-trefoil superfamily. Structural comparison and simulation suggest that the NTD has a putative lipid-binding cavity, whereas the CTD has a potential sugar-binding site. However, we were unable to detect the binding of either lipid or sugar. Therefore, further investigations are needed to characterize the molecular function of this protein.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei Anhui 230027, People's Republic of China.