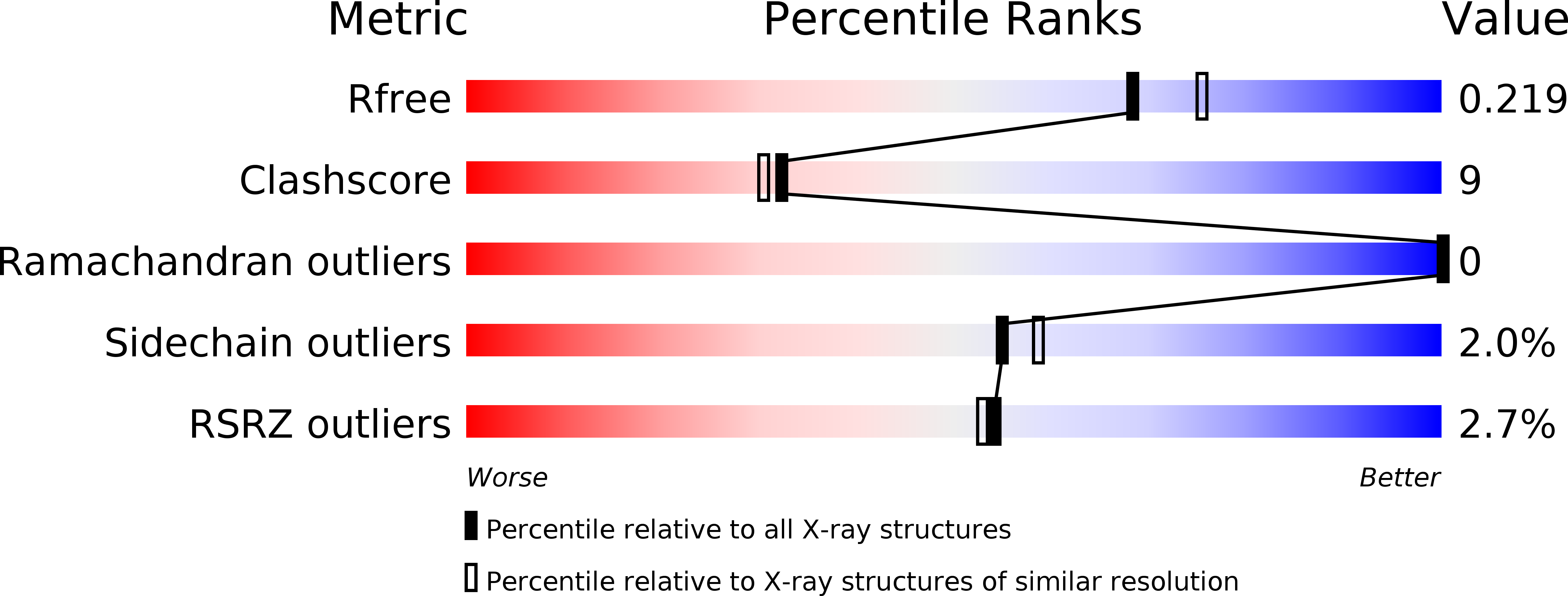
Structural mechanism of demethylation and inactivation of protein phosphatase 2A.
Xing, Y., Li, Z., Chen, Y., Stock, J.B., Jeffrey, P.D., Shi, Y.(2008) Cell 133: 154-163
- PubMed: 18394995
- DOI: https://doi.org/10.1016/j.cell.2008.02.041
- Primary Citation of Related Structures:
3C5V, 3C5W - PubMed Abstract:
Protein phosphatase 2A (PP2A) is an important serine/threonine phosphatase that plays a role in many biological processes. Reversible carboxyl methylation of the PP2A catalytic subunit is an essential regulatory mechanism for its function. Demethylation and negative regulation of PP2A is mediated by a PP2A-specific methylesterase PME-1, which is conserved from yeast to humans. However, the underlying mechanism of PME-1 function remains enigmatic. Here we report the crystal structures of PME-1 by itself and in complex with a PP2A heterodimeric core enzyme. The structures reveal that PME-1 directly binds to the active site of PP2A and that this interaction results in the activation of PME-1 by rearranging the catalytic triad into an active conformation. Strikingly, these interactions also lead to inactivation of PP2A by evicting the manganese ions that are required for the phosphatase activity of PP2A. These observations identify a dual role of PME-1 that regulates PP2A activation, methylation, and holoenzyme assembly in cells.
Organizational Affiliation:
Department of Molecular Biology, Princeton University, Lewis Thomas Laboratory, Princeton, NJ 08544, USA.