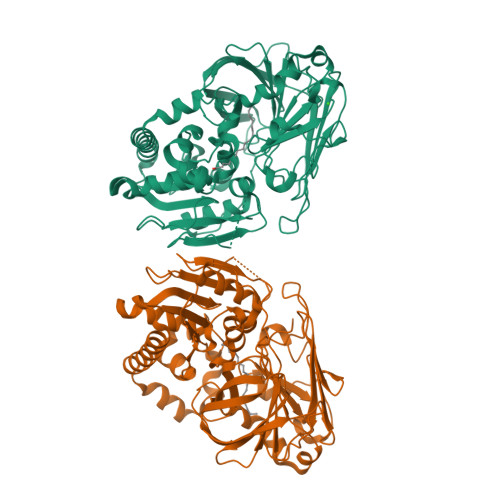
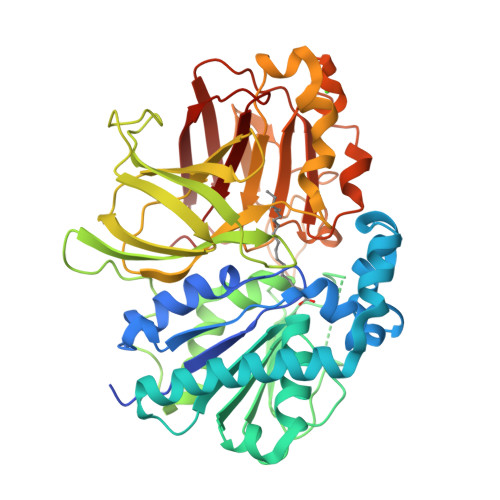
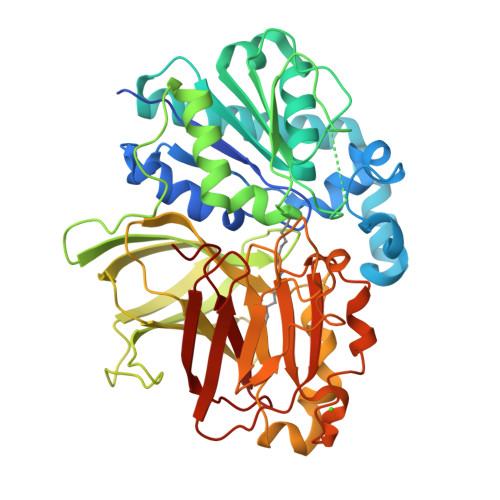
Structure of the alkalohyperthermophilic Archaeoglobus fulgidus lipase contains a unique C-terminal domain essential for long-chain substrate binding.
Chen, C.K., Lee, G.C., Ko, T.P., Guo, R.T., Huang, L.M., Liu, H.J., Ho, Y.F., Shaw, J.F., Wang, A.H.(2009) J Mol Biology 390: 672-685
- PubMed: 19447113
- DOI: https://doi.org/10.1016/j.jmb.2009.05.017
- Primary Citation of Related Structures:
2ZYH, 2ZYI, 2ZYR, 2ZYS - PubMed Abstract:
Several crystal structures of AFL, a novel lipase from the archaeon Archaeoglobus fulgidus, complexed with various ligands, have been determined at about 1.8 A resolution. This enzyme has optimal activity in the temperature range of 70-90 degrees C and pH 10-11. AFL consists of an N-terminal alpha/beta-hydrolase fold domain, a small lid domain, and a C-terminal beta-barrel domain. The N-terminal catalytic domain consists of a 6-stranded beta-sheet flanked by seven alpha-helices, four on one side and three on the other side. The C-terminal lipid binding domain consists of a beta-sheet of 14 strands and a substrate covering motif on top of the highly hydrophobic substrate binding site. The catalytic triad residues (Ser136, Asp163, and His210) and the residues forming the oxyanion hole (Leu31 and Met137) are in positions similar to those of other lipases. Long-chain lipid is located across the two domains in the AFL-substrate complex. Structural comparison of the catalytic domain of AFL with a homologous lipase from Bacillus subtilis reveals an opposite substrate binding orientation in the two enzymes. AFL has a higher preference toward long-chain substrates whose binding site is provided by a hydrophobic tunnel in the C-terminal domain. The unusually large interacting surface area between the two domains may contribute to thermostability of the enzyme. Two amino acids, Asp61 and Lys101, are identified as hinge residues regulating movement of the lid domain. The hydrogen-bonding pattern associated with these two residues is pH dependent, which may account for the optimal enzyme activity at high pH. Further engineering of this novel lipase with high temperature and alkaline stability will find its use in industrial applications.
Organizational Affiliation:
Institute of Biochemical Sciences, National Taiwan University, Taipei, Taiwan.