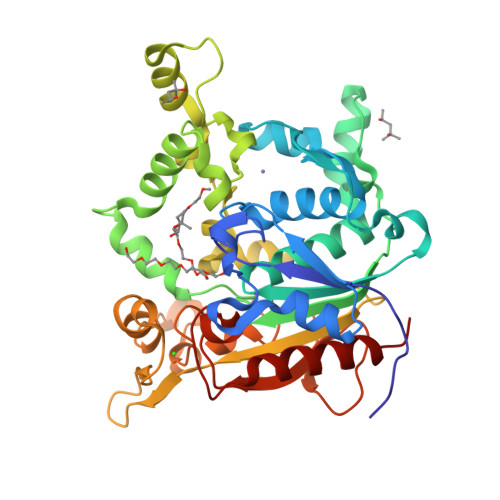
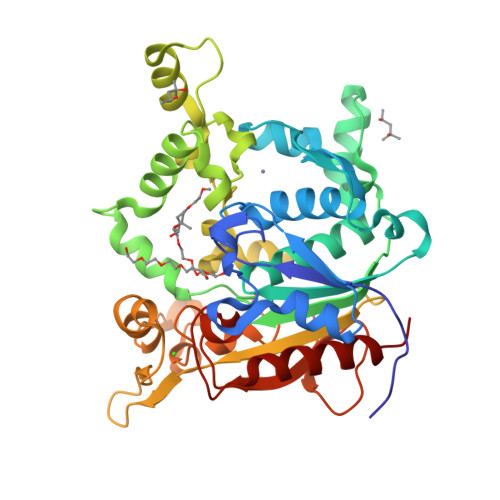
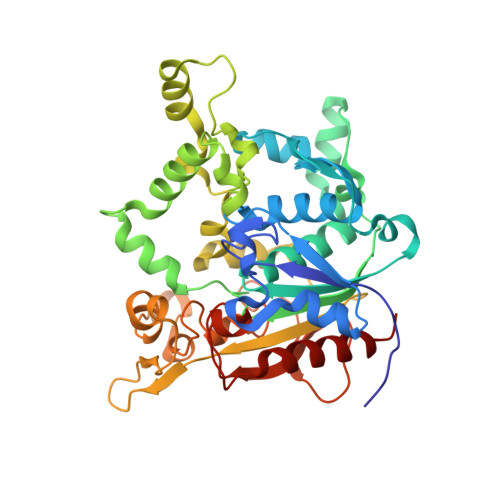
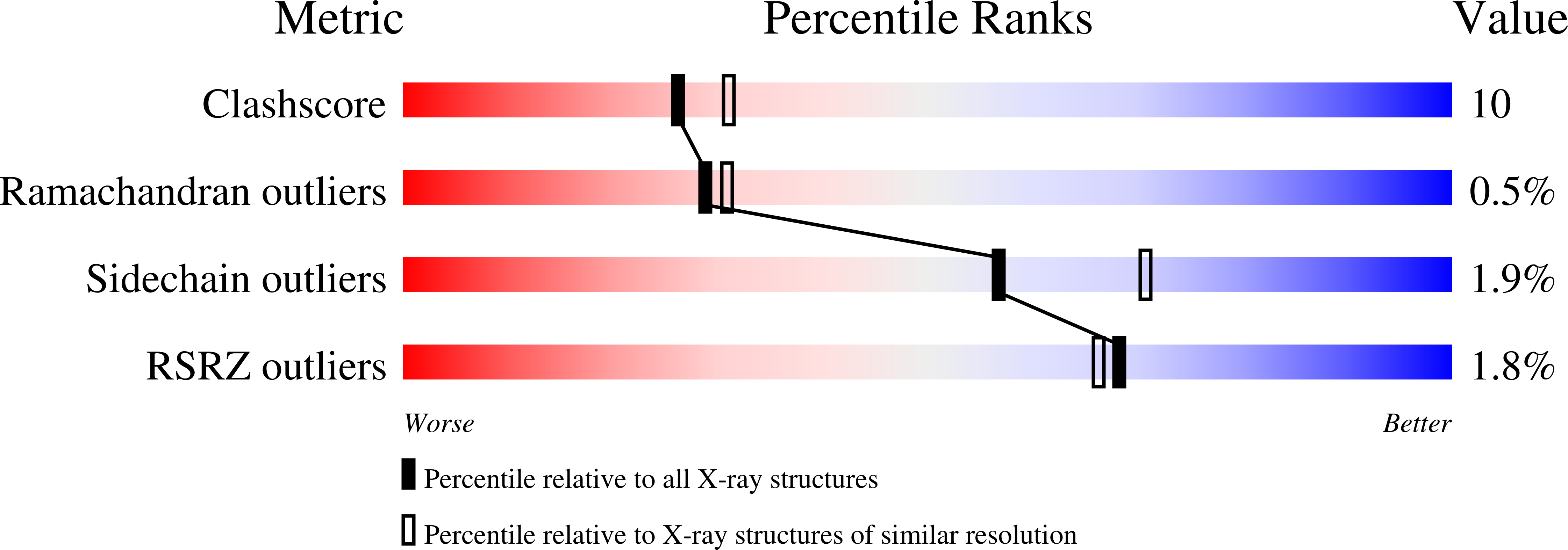Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements.
Carrasco-Lopez, C., Godoy, C., de Las Rivas, B., Fernandez-Lorente, G., Palomo, J.M., Guisan, J.M., Fernandez-Lafuente, R., Martinez-Ripoll, M., Hermoso, J.A.(2009) J Biological Chem 284: 4365-4372
- PubMed: 19056729
- DOI: https://doi.org/10.1074/jbc.M808268200
- Primary Citation of Related Structures:
2W22 - PubMed Abstract:
The bacterial thermoalkalophilic lipases that hydrolyze saturated fatty acids at 60-75 degrees C and pH 8-10 are grouped as the lipase family I.5. We report here the crystal structure of the lipase from Geobacillus thermocatenulatus, the first structure of a member of the lipase family I.5 showing an open configuration. Unexpectedly, enzyme activation involves large structural rearrangements of around 70 amino acids and the concerted movement of two lids, the alpha6- and alpha7-helices, unmasking the active site. Central in the restructuring process of the lids are both the transfer of bulky hydrophobic residues out of the N-terminal end of the alpha6-helix and the incorporation of short side chain residues to the alpha6 C-terminal end. All these structural changes are stabilized by the Zn(2+)-binding domain, which is characteristic of this family of lipases. Two detergent molecules are placed in the active site, mimicking chains of the triglyceride substrate, demonstrating the position of the oxyanion hole and the three pockets that accommodate the sn-1, sn-2, and sn-3 fatty acids chains. The combination of structural and biochemical studies indicate that the lid opening is not mediated by temperature but triggered by interaction with lipid substrate.
Organizational Affiliation:
Grupo de Cristalografía Macromolecular y Biología Estructural, Instituto de Química-Física Rocasolano, CSIC, Serrano 119, 28006-Madrid, Spain.