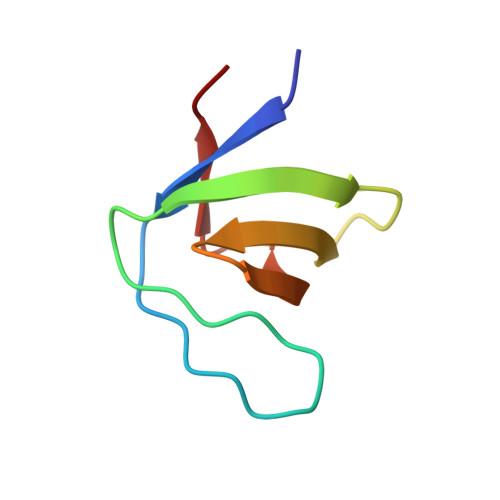
Distinct Binding Modes of Two Epitopes in Gab2 that Interact with the Sh3C Domain of Grb2.
Harkiolaki, M., Tsirka, T., Lewitzky, M., Simister, P.C., Joshi, D., Bird, L.E., Jones, E.Y., O'Reilly, N., Feller, S.M.(2009) Structure 17: 809
- PubMed: 19523899
- DOI: https://doi.org/10.1016/j.str.2009.03.017
- Primary Citation of Related Structures:
2VVK, 2VWF, 2W0Z, 2W10 - PubMed Abstract:
Grb2 and Gab2 form a complex implicated in normal cell signaling and cancer development. Binding of the Grb2SH3C domain to Gab2 is essential for the interaction, but molecular details remained undefined. Using peptide arrays and isothermal titration calorimetry, two Grb2SH3C binding sites in Gab2 (Gab2a and Gab2b) were confirmed and characterized. Gab2a bears similarity to a p27Kip1 epitope that also binds Grb2SH3C. Crystal structures of both Gab2 epitopes complexed with Grb2SH3C reveal that Gab2b contains a 3(10) helix that positions the arginine and lysine of the core-binding motif RxxK in parallel orientation. In contrast, the Gab2a RxxK motif is embedded in a PPII helix with Arg and Lys in staggered orientation. A similar interaction mode is also present in a new complex of Mona/GadsSH3C with an RxxxxK epitope from the putative phosphatase HD-PTP. In summary, our study reveals interaction types of SH3 domains, highlighting their great versatility.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford OX37BN, UK.