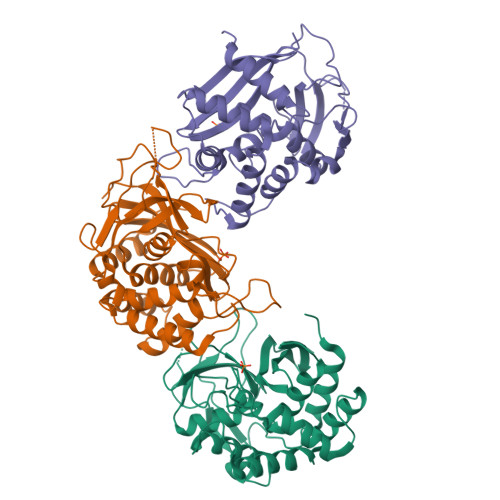
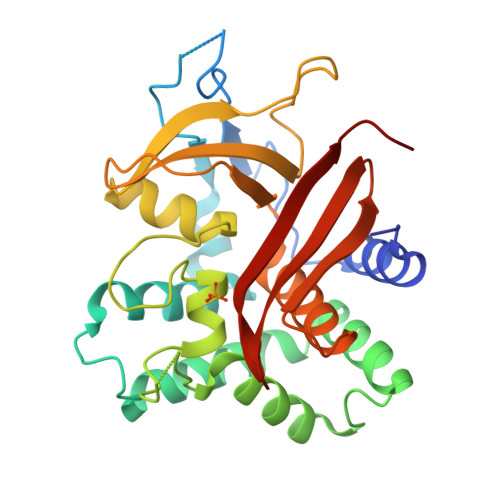
The Structure of the Endoribonuclease Xendou: From Small Nucleolar RNA Processing to Severe Acute Respiratory Syndrome Coronavirus Replication.
Renzi, F., Caffarelli, E., Laneve, P., Bozzoni, I., Brunori, M., Vallone, B.(2006) Proc Natl Acad Sci U S A 103: 12365
- PubMed: 16895992
- DOI: https://doi.org/10.1073/pnas.0602426103
- Primary Citation of Related Structures:
2C1W - PubMed Abstract:
Small nucleolar RNAs (snoRNAs) play a key role in eukaryotic ribosome biogenesis. In most cases, snoRNAs are encoded in introns and are released through the splicing reaction. Some snoRNAs are, instead, produced by an alternative pathway consisting of endonucleolytic processing of pre-mRNA. XendoU, the endoribonuclease responsible for this activity, is a U-specific, metal-dependent enzyme that releases products with 2'-3' cyclic phosphate termini. XendoU is broadly conserved among eukaryotes, and it is a genetic marker of nidoviruses, including the severe acute respiratory syndrome coronavirus, where it is essential for replication and transcription. We have determined by crystallography the structure of XendoU that, by refined search methodologies, appears to display a unique fold. Based on sequence conservation, mutagenesis, and docking simulations, we have identified the active site. The conserved structural determinants of this site may provide a framework for attempting to design antiviral drugs to interfere with the infectious nidovirus life cycle.
Organizational Affiliation:
Istituto di Biologia e Patologia Molecolari del Consiglio Nazionale delle Ricerche, 00185 Rome, Italy.