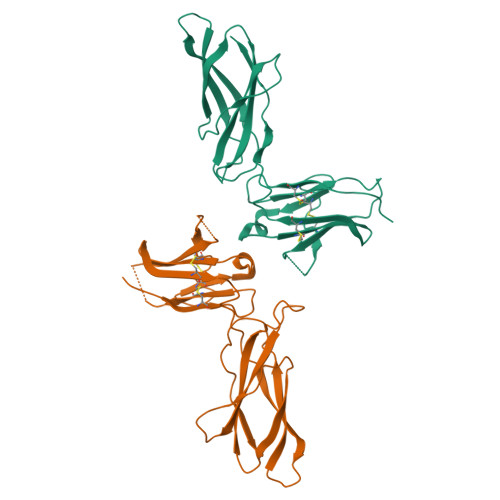
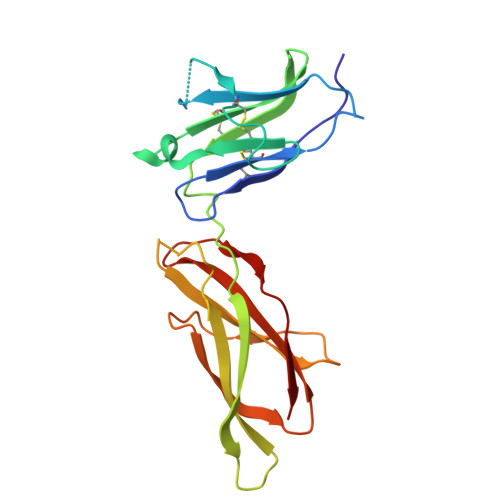
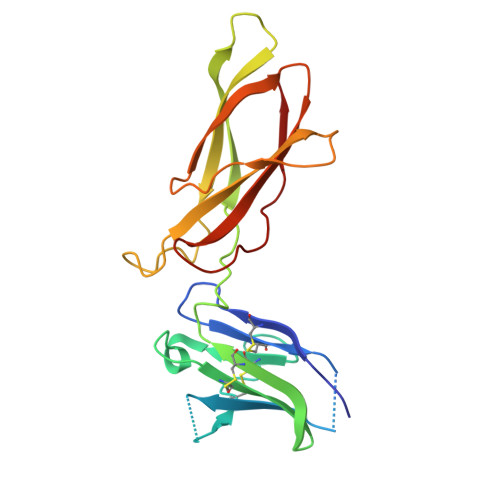
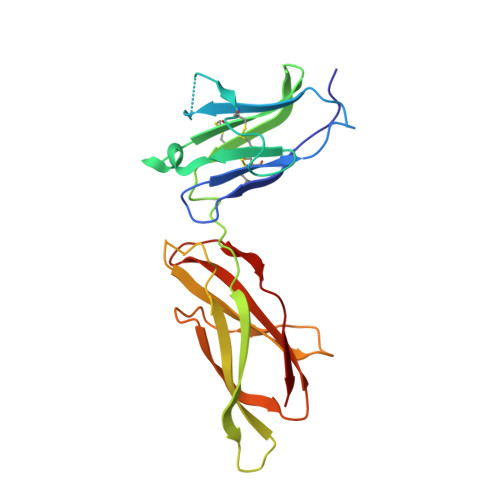
Model for growth hormone receptor activation based on subunit rotation within a receptor dimer.
Brown, R.J., Adams, J.J., Pelekanos, R.A., Wan, Y., McKinstry, W.J., Palethorpe, K., Seeber, R.M., Monks, T.A., Eidne, K.A., Parker, M.W., Waters, M.J.(2005) Nat Struct Mol Biol 12: 814-821
- PubMed: 16116438
- DOI: https://doi.org/10.1038/nsmb977
- Primary Citation of Related Structures:
2AEW - PubMed Abstract:
Growth hormone is believed to activate the growth hormone receptor (GHR) by dimerizing two identical receptor subunits, leading to activation of JAK2 kinase associated with the cytoplasmic domain. However, we have reported previously that dimerization alone is insufficient to activate full-length GHR. By comparing the crystal structure of the liganded and unliganded human GHR extracellular domain, we show here that there is no substantial change in its conformation on ligand binding. However, the receptor can be activated by rotation without ligand by inserting a defined number of alanine residues within the transmembrane domain. Fluorescence resonance energy transfer (FRET), bioluminescence resonance energy transfer (BRET) and coimmunoprecipitation studies suggest that receptor subunits undergo specific transmembrane interactions independent of hormone binding. We propose an activation mechanism involving a relative rotation of subunits within a dimeric receptor as a result of asymmetric placement of the receptor-binding sites on the ligand.
Organizational Affiliation:
Institute for Molecular Bioscience and School of Biomedical Sciences, University of Queensland, St. Lucia, Queensland, Australia.