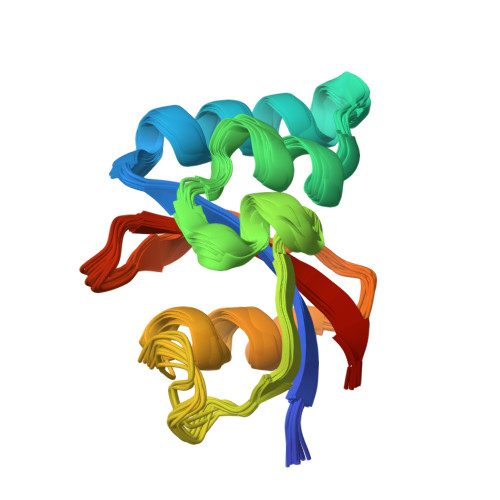
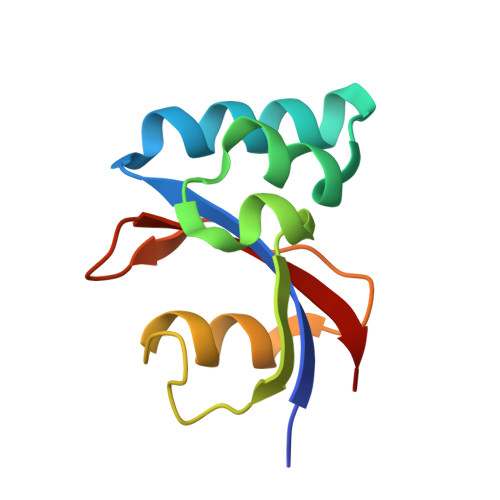
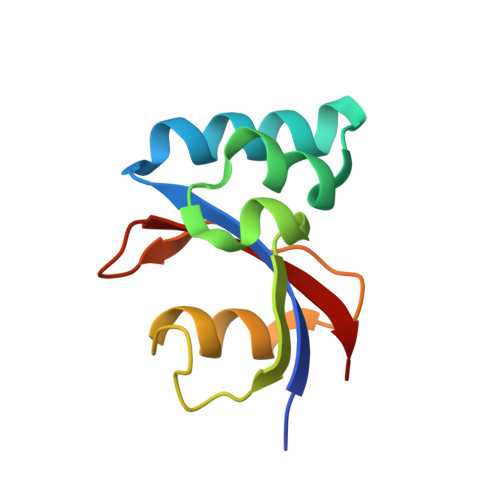
NMR solution structure and characterization of substrate binding site of the PPIase domain of PrsA protein from Bacillus subtilis
Tossavainen, H., Permi, P., Purhonen, S.L., Sarvas, M., Kilpelainen, I., Seppala, R.(2006) FEBS Lett 580: 1822-1826
- PubMed: 16516208
- DOI: https://doi.org/10.1016/j.febslet.2006.02.042
- Primary Citation of Related Structures:
1ZK6 - PubMed Abstract:
PrsA is a peptidyl-prolyl isomerase (PPIase) from Bacillus subtilis belonging to the parvulin family of PPIases. It is a membrane bound lipoprotein at the membrane-wall interface, involved in folding of exported proteins. We present the NMR solution structure of the PPIase domain of PrsA, the first from a Gram-positive bacterium. In addition we mapped out the active site with NMR titration experiments. A high degree of conservation with other members of the parvulin family was revealed in the structure and binding site. Interactions with substrate peptides were also characterized by mutated domains revealing that H122 is indispensable for overall correct folding.
Organizational Affiliation:
NMR Laboratory, Institute of Biotechnology, Viikinkaari 1, P.O. Box 65, FI-00014, University of Helsinki, Helsinki, Finland.