Crystal structure and mutational analysis of the Saccharomyces cerevisiae cell cycle regulatory protein Cks1: implications for domain swapping, anion binding and protein interactions.
Bourne, Y., Watson, M.H., Arvai, A.S., Bernstein, S.L., Reed, S.I., Tainer, J.A.(2000) Structure 8: 841-850
- PubMed: 10997903
- DOI: https://doi.org/10.1016/s0969-2126(00)00175-1
- Primary Citation of Related Structures:
1QB3 - PubMed Abstract:
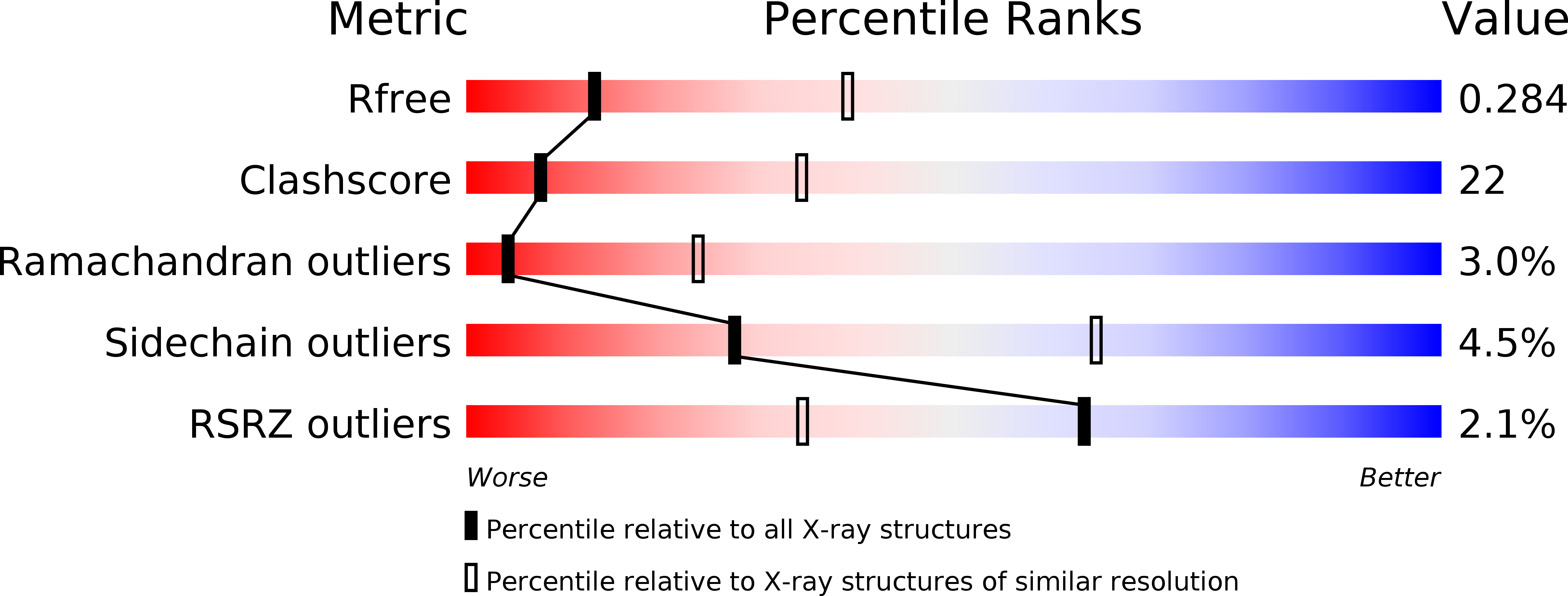
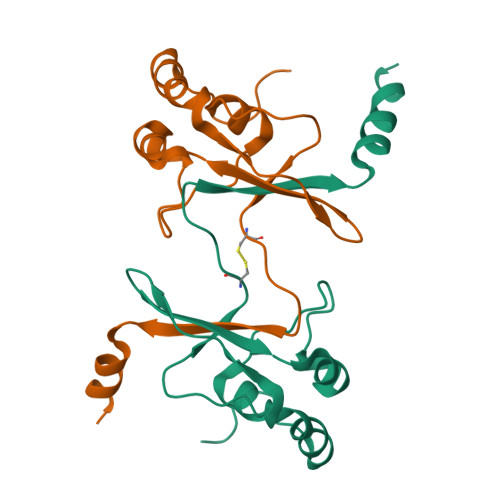
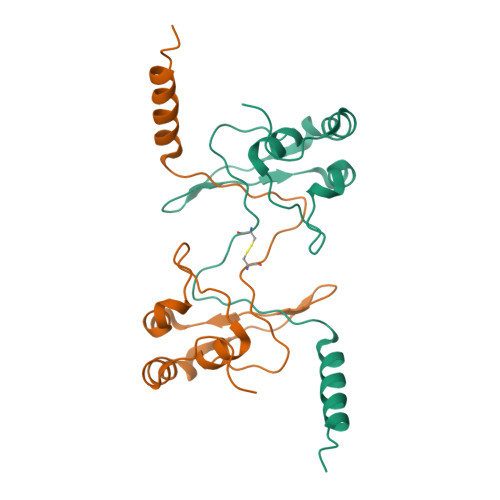
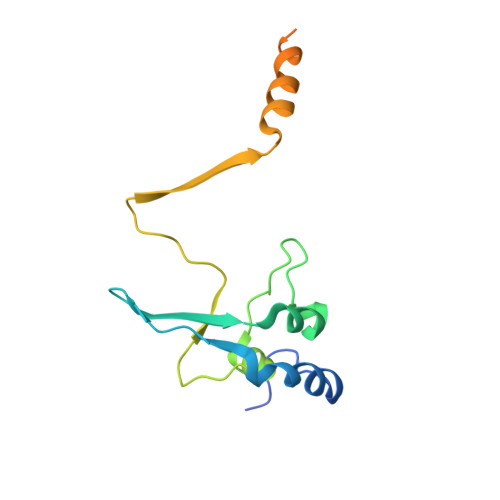
The Saccharomyces cerevisiae protein Cks1 (cyclin-dependent kinase subunit 1) is essential for cell-cycle progression. The biological function of Cks1 can be modulated by a switch between two distinct molecular assemblies: the single domain fold, which results from the closing of a beta-hinge motif, and the intersubunit beta-strand interchanged dimer, which arises from the opening of the beta-hinge motif. The crystal structure of a cyclin-dependent kinase (Cdk) in complex with the human Cks homolog CksHs1 single-domain fold revealed the importance of conserved hydrophobic residues and charged residues within the beta-hinge motif. The 3.0 A resolution Cks1 structure reveals the strict structural conservation of the Cks alpha/beta-core fold and the beta-hinge motif. The beta hinge identified in the Cks1 structure includes a novel pivot and exposes a cluster of conserved tyrosine residues that are involved in Cdk binding but are sequestered in the beta-interchanged Cks homolog suc1 dimer structure. This Cks1 structure confirms the conservation of the Cks anion-binding site, which interacts with sidechain residues from the C-terminal alpha helix of another subunit in the crystal. The Cks1 structure exemplifies the conservation of the beta-interchanged dimer and the anion-binding site in evolutionarily distant yeast and human Cks homologs. Mutational analyses including in vivo rescue of CKS1 disruption support the dual functional roles of the beta-hinge residue Glu94, which participates in Cdk binding, and of the anion-binding pocket that is located 22 A away and on an opposite face to Glu94. The Cks1 structure suggests a biological role for the beta-interchanged dimer and the anion-binding site in targeting Cdks to specific phosphoproteins during cell-cycle progression.
Organizational Affiliation:
Centre National de la Recherche Scientifique, Marseille, France. yves@afmb.cnrs-mrs.fr