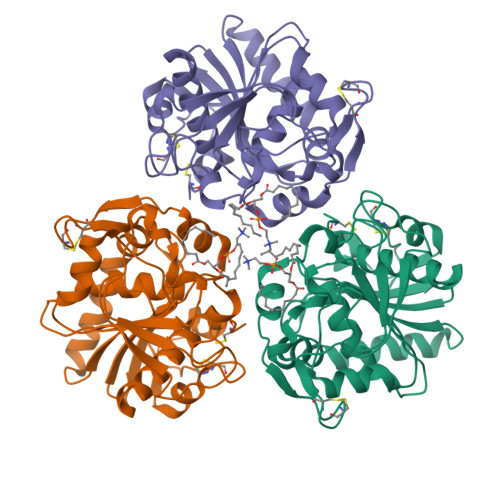
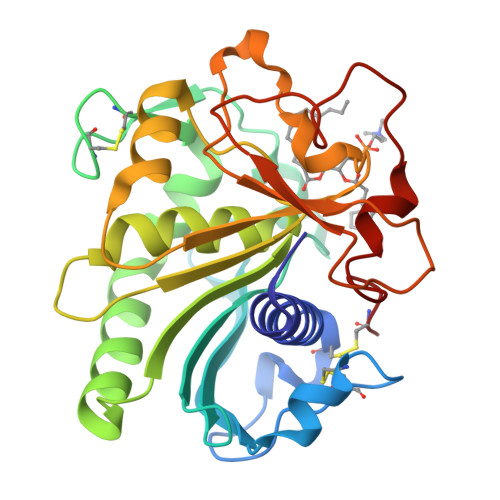
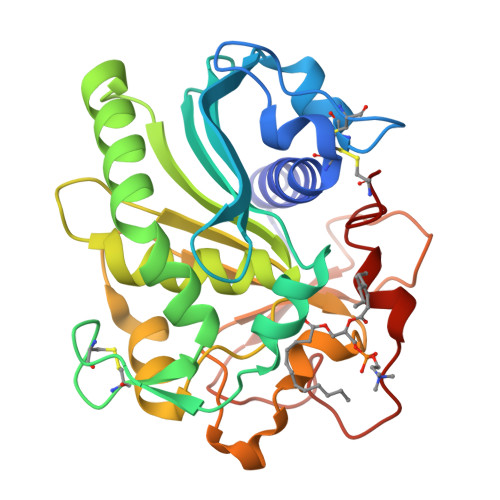
Structural origins of the interfacial activation in Thermomyces (Humicola) lanuginosa lipase.
Brzozowski, A.M., Savage, H., Verma, C.S., Turkenburg, J.P., Lawson, D.M., Svendsen, A., Patkar, S.(2000) Biochemistry 39: 15071-15082
- PubMed: 11106485
- DOI: https://doi.org/10.1021/bi0013905
- Primary Citation of Related Structures:
1DT3, 1DT5, 1DTE, 1DU4, 1EIN - PubMed Abstract:
The already known X-ray structures of lipases provide little evidence about initial, discrete structural steps occurring in the first phases of their activation in the presence of lipids (process referred to as interfacial activation). To address this problem, five new Thermomyces (formerly Humicola) lanuginosa lipase (TlL) crystal structures have been solved and compared with four previously reported structures of this enzyme. The bias coming from different crystallization media has been minimized by the growth of all crystals under the same crystallization conditions, in the presence of detergent/lipid analogues, with low or high ionic strength as the only main variable. Resulting structures and their characteristic features allowed the identification of three structurally distinct species of this enzyme: low activity form (LA), activated form (A), and fully Active (FA) form. The isomerization of the Cys268-Cys22 disulfide, synchronized with the formation of a new, short alpha(0) helix and flipping of the Arg84 (Arginine switch) located in the lid's proximal hinge, have been postulated as the key, structural factors of the initial transitions between LA and A forms. The experimental results were supplemented by theoretical calculations. The magnitude of the activation barrier between LA (ground state) and A (end state) forms of TlL (10.6 kcal/mol) is comparable to the enthalpic barriers typical for ring flips and disulfide isomerizations at ambient temperatures. This suggests that the sequence of the structural changes, as exemplified in various TlL crystal structures, mirror those that may occur during interfacial activation.
Organizational Affiliation:
York Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, YO10 5DD, U.K. marek@ysbl.york.ac.uk