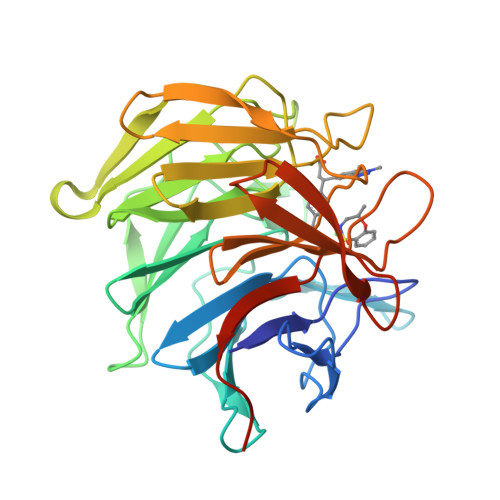
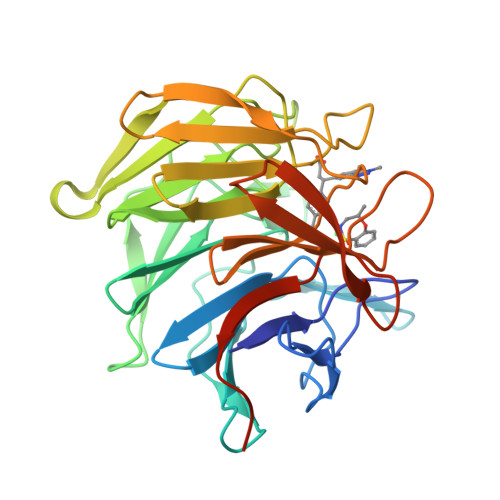
Mono-Acidic Inhibitors of the Kelch-Like Ech-Associated Protein 1 : Nuclear Factor Erythroid 2-Related Factor 2 (Keap1:Nrf2) Protein-Protein Interaction with High Cell Potency Identified by Fragment-Based Discovery.
Davies, T.G., Wixted, W.E., Coyle, J.E., Griffiths-Jones, C., Hearn, K., Mcmenamin, R.L., Norton, D., Rich, S.J., Richardson, C., Saxty, G., Willems, H.M.G., Woolford, A.J., Cottom, J.E., Kou, J., Yonchuk, J.G., Feldser, H.G., Sanchez, Y., Foley, J.P., Bolognese, B.J., Logan, G.A., Podolin, P.L., Yan, H., Callahan, J.F., Heightman, T.D., Kerns, J.K.(2016) J Med Chem 59: 3991
- PubMed: 27031670
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00228
- Primary Citation of Related Structures:
5FNQ, 5FNR, 5FNS, 5FNT, 5FNU, 5FZJ, 5FZN - PubMed Abstract:
KEAP1 is the key regulator of the NRF2-mediated cytoprotective response, and increasingly recognized as a target for diseases involving oxidative stress. Pharmacological intervention has focused on molecules that decrease NRF2-ubiquitination through covalent modification of KEAP1 cysteine residues, but such electrophilic compounds lack selectivity and may be associated with off-target toxicity. We report here the first use of a fragment-based approach to directly target the KEAP1 Kelch-NRF2 interaction. X-ray crystallographic screening identified three distinct "hot-spots" for fragment binding within the NRF2 binding pocket of KEAP1, allowing progression of a weak fragment hit to molecules with nanomolar affinity for KEAP1 while maintaining drug-like properties. This work resulted in a promising lead compound which exhibits tight and selective binding to KEAP1, and activates the NRF2 antioxidant response in cellular and in vivo models, thereby providing a high quality chemical probe to explore the therapeutic potential of disrupting the KEAP1-NRF2 interaction.
Organizational Affiliation:
Astex Pharmaceuticals, 436 Cambridge Science Park, Cambridge CB4 0QA, U.K.