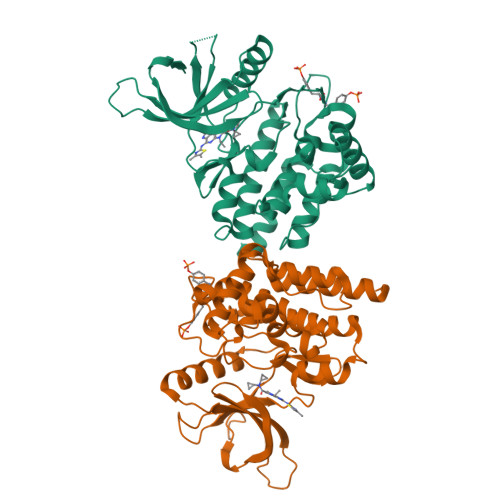
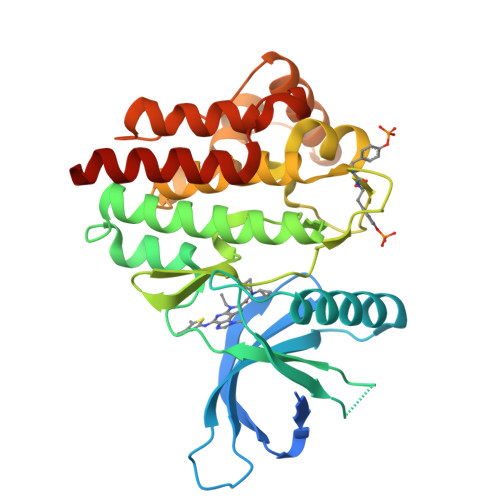
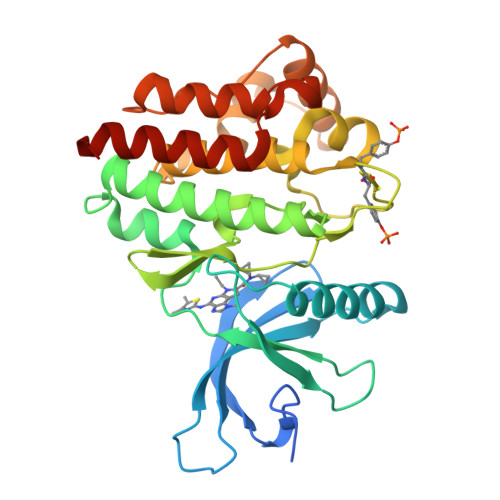
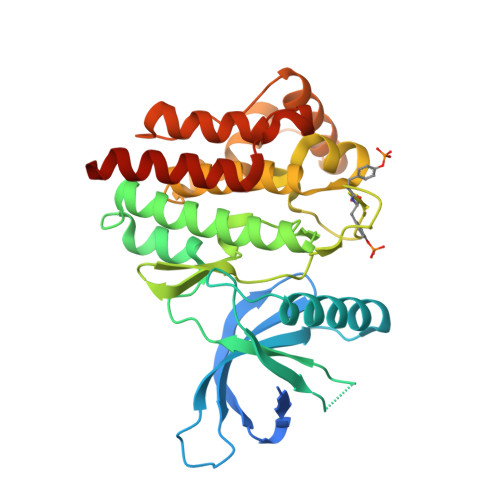
Structure-Based Design of Selective Janus Kinase 2 Imidazo[4,5-d]pyrrolo[2,3-b]pyridine Inhibitors.
Hart, A.C., Schroeder, G.M., Wan, H., Grebinski, J., Inghrim, J., Kempson, J., Guo, J., Pitts, W.J., Tokarski, J.S., Sack, J.S., Khan, J.A., Lippy, J., Lorenzi, M.V., You, D., McDevitt, T., Vuppugalla, R., Zhang, Y., Lombardo, L.J., Trainor, G.L., Purandare, A.V.(2015) ACS Med Chem Lett 6: 845-849
- PubMed: 26288682
- DOI: https://doi.org/10.1021/acsmedchemlett.5b00225
- Primary Citation of Related Structures:
5CF4, 5CF5, 5CF6 - PubMed Abstract:
Early hit to lead work on a pyrrolopyridine chemotype provided access to compounds with biochemical and cellular potency against Janus kinase 2 (JAK2). Structure-based drug design along the extended hinge region of JAK2 led to the identification of an important H-bond interaction with the side chain of Tyr 931, which improved JAK family selectivity. The 4,5-dimethyl thiazole analogue 18 demonstrated high levels of JAK family selectivity and was identified as a promising lead for the program.
Organizational Affiliation:
Bristol-Myers Squibb Research & Development , Princeton, New Jersey 08543, United States.